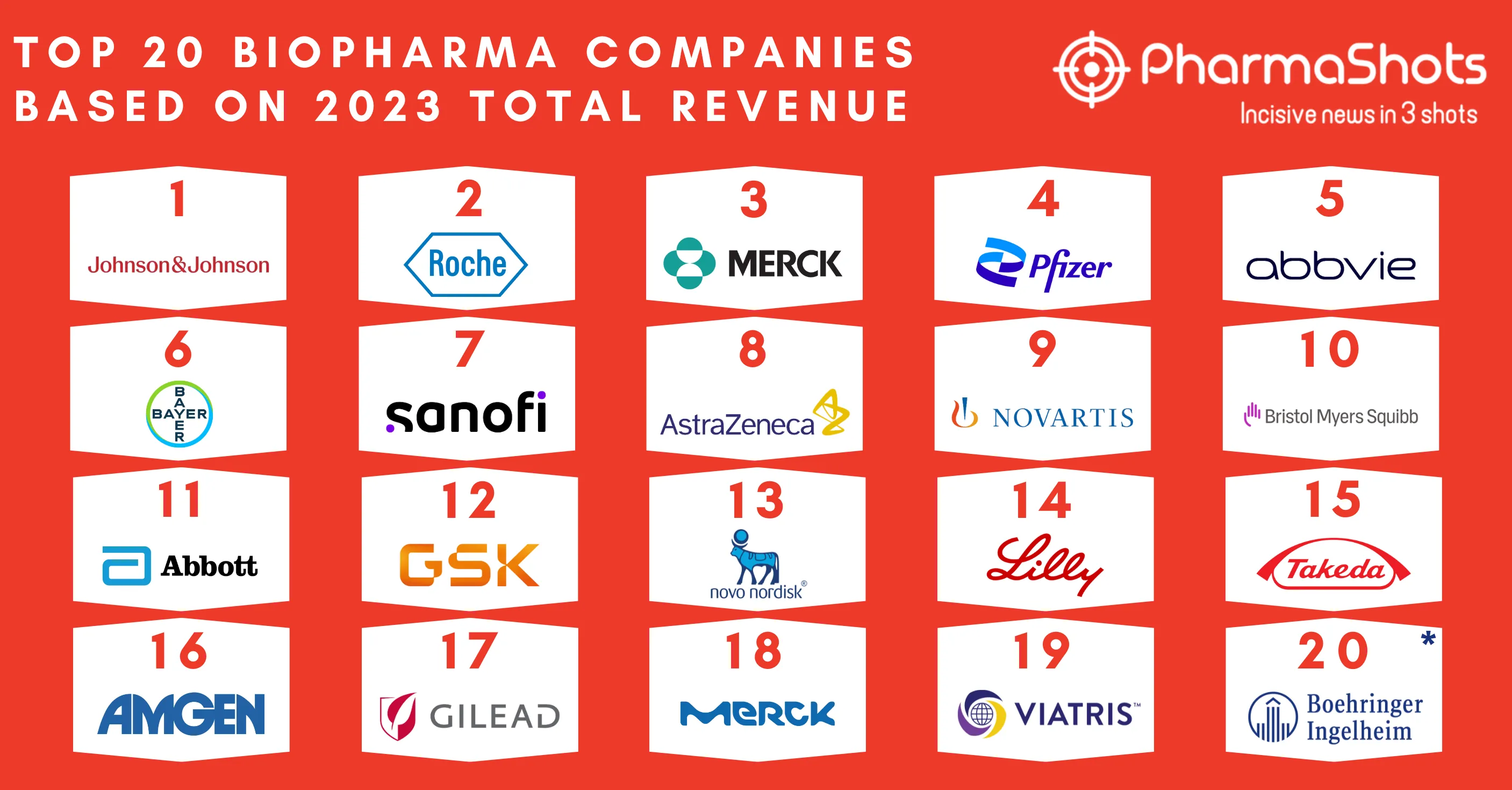
Top 20 Biopharma Deal Terminations of 2021 Based on Total Deal Value
Shots:
- The biopharma industry saw numerous deal terminations in 2021. Clinical and regulatory results, change of control limitations, and strategic reprioritizations were among the most common reasons for deal termination.
- SOBI and Advent's acquisition agreement in 2021 ranked first under which Advent International offered to acquire SOBI. The second position goes to Galapagos’ development and commercialization deal with Gilead for a P-III drug candidate.
- This article is based on the 2021 biopharma deal data provided by Chris Dokomajilar of DealForma. PharmaShots compiled a list of the top 20 terminations of 2021 based on total deal value.
.png)
Cytokinetics terminated its development and commercialization deal with Astellas
In Apr 2020, Cytokinetics granted Astellas exclusive rights to co-develop and commercialize reldesemtiv, CK-601, and other FSRA compounds. Upon mutual acceptance, Cytokinetics had an exclusive option to co-promote certain products in the US, Canada, and/or Europe. Astellas will reimburse promotion costs. Astellas was responsible for all development costs, and Cytokinetics had the exclusive right to co-fund certain early-stage development. Cytokinetics was eligible to receive up to $25M to $35M per product or approximately up to $250M in regulatory milestones and up to $200M in sales milestones, plus mid-single to low double-digit royalties. If Cytokinetics also had an option to co-fund the development costs, it was eligible to receive mid-to-high double-digit royalties. In May 2021, Astellas terminated the agreement, and Cytokinetics regained worldwide rights.
.png)
MEI Pharma terminated its development and commercialization deal with Helsinn Group
In Aug 2016, MEI Pharma granted Helsinn Group exclusive rights to develop and commercialize Pracinostat, a P-III drug candidate for treating acute myeloid leukemia (AML) and other indications. Helsinn was responsible for development funding. Under the agreement, Helsinn and MEI Pharma collaborated to explore an optimal dosing regimen of Pracinostat in combination with azacitidine to treat high-risk MDS. Helsinn agreed to make a $5M equity investment. MEI Pharma received a $15M upfront and a $5M payment upon dosing of the first patient. Additionally, MEI Pharma was eligible to receive up to $444M in development, regulatory, and sales-based milestone payments plus royalties. In Nov 2021, MEI and Helsinn mutually terminated the agreement due to the discontinuation of Pracinostat.
.png)
Khosla Ventures Acquisition Corp. terminated its reverse merger with Valo Health
In Jun 2021, Khosla Ventures Acquisition Corp. signed an agreement to reverse merge with Valo Health in a SPAC transaction. The deal gave Valo $333M from the SPAC and a $168.5M PIPE financing for a pro forma market value of approx. $2.8B with a cash balance of $750M, including existing Valo cash, the gross PIPE proceeds, and the net cash was held in KVAC's trust, assuming no redemptions. In Nov 2021, Khosla Ventures Acquisition Corp and Valo Health mutually terminated the agreement.
.png)
CureVac terminated its development and commercialization deal with Boehringer Ingelheim
In Sep 2014, CureVac granted Boehringer Ingelheim exclusive rights to develop and commercialize BI 1361849 (formerly CV9202), a therapeutic mRNA vaccine based on CureVac's mRNA technology RNActive for the treatment of lung cancer. BI was responsible for the clinical investigation of CV9202 in combination with afatinib in patients with advanced or EGFR mutated non-small cell lung cancer and chemo-radiation therapy in patients with unresectable stage III NSCLC. CureVac received $45M upfront and was eligible to receive up to $556M in milestone payments, plus royalties. In Aug 2021, CureVac terminated the agreement with BI effective 17 Nov 2021.
.png)
Bristol-Myers Squibb terminated its development and commercialization deal with Biogen
In Apr 2017, BMS granted Biogen exclusive rights to develop and commercialize BMS-986168, a P-II molecule to treat Alzheimer's disease (AD) and progressive supranuclear palsy (PSP). BMS received $300M upfront and was eligible to receive up to $410M in milestone payments plus royalties. Biogen was responsible for the full development and global commercialization of BMS-986168 in AD and PSP. Biogen assumes all remaining obligations to the former stockholders of iPierian, which BMS acquired in 2014. In Jun 2021, Biogen terminated the agreement as the drug failed to meet its primary efficacy endpoints.
.png)
Inovio terminated its development and commercialization deal with MedImmune
In Aug 2015, Inovio Pharmaceuticals granted MedImmune exclusive rights to develop and commercialize INO-3112, a human papillomavirus (HPV) immunotherapy targeting cancers caused by HPV types 16 and 18. MedImmune had the intention to study INO-3112 in combination with certain immunotherapy from its pipeline. Inovio Pharmaceuticals received $27.5M upfront and was eligible to receive up to $700M in milestones, plus royalties. MedImmune agreed to fund all development costs, and Inovio was entitled to receive up to double-digit tiered royalties on INO-3112 product sales. Additionally, MedImmune was to develop and commercialize up to two DNA-based cancer vaccines not included in Inovio's product pipeline. Inovio Pharmaceuticals was also eligible to receive development, regulatory, and commercialization milestone payments. In Nov 2021, Inovio Pharmaceuticals and MedImmune terminated the agreement.
.png)
Aduro terminated its development and commercialization deal with Novartis
In Mar 2015, Aduro granted Novartis worldwide rights to develop and commercialize STING (Stimulator of Interferon Genes) receptor-targeted ADU-S100, cyclic dinucleotide (CDN) for the treatment of cancer. Aduro was responsible for commercialization activities and book sales in the US, with Novartis leading commercialization and recognizing sales in the rest of the world. Aduro received $200M upfront and, in 2016, earning up to a $35M development milestone upon initiation of a P-I trial for the first STING product candidate. Aduro was also eligible to receive up to an additional $215M in development milestones and up to another $250M in regulatory approval milestones, plus royalties. Aduro would fund 38% of the development cost, and Novartis would fund 62%. Both companies were to share profits based on sales with 50% in the US and 45% for Japan and other major European countries. In addition, companies would have the right to opt-out of the early clinical trials and join the pivotal study by reimbursing the development cost. In Apr 2021, Novartis terminated the agreement following its recent merger with Chinook Therapeutics.
.png)
C4 Therapeutics terminated its Option and License Deal with Roche
In Jan 2016, C4 Therapeutics(C4T) granted Roche an option to license exclusive, worldwide rights for targeted protein degradation (TPD) therapies developed using C4's Degronimid technology. C4T had an option to co-develop and co-promote some programs in the US. C4 Therapeutics received a $15M upfront- $1 to $4M as target initiation fees with the potential deal value exceeding $750M. In Dec 2018, C4T and Roche restated the agreement, granting C4T a more active role in manufacturing and commercialization targets. C4T would have opt-in for co-development and co-detailing in exchange for a higher profit share. The companies revised the target structure with three assigned during the restated agreement and would be assigned three more in the future. C4T received an additional $40M upfront and - $1M each in R&D fee annually. C4T will receive $7M to $12M if it passes the GLP toxicology studies and $20M if it progresses through P-I. Roche was required to pay $2M and $3M upon identifying a lead series and the commencement of GLP toxicology studies. C4T was also eligible to receive up to $275M in research, development, and commercial milestone payments per target plus royalties. In Nov 2021, C4T and Roche mutually terminated the agreement, allowing C4T to advance CFT1946 and any other BRAF degraders independently from Roche.
.png)
Theravance terminated its development and commercialization with Janssen
In Feb 2018, Theravance granted Janssen an option to license exclusive worldwide rights to TD-1473 and related backup compounds for Crohn's disease and ulcerative colitis. Theravance received a $100M upfront and was eligible to receive up to $900M in additional payments if Janssen opted to license. Janssen had an option to gain the exclusive rights by paying Theravance Biopharma $200M, followed by the completion of P-II Crohn's study and the P-IIb induction portion of the ulcerative colitis study. Theravance had the exclusive option to co-commercialize TD-1473 in the US, and Janssen was solely responsible for the rest of the world. The partners were to share costs and profits (67% Janssen/33% Theravance) if Theravance elected to co-develop. Theravance was also eligible for undisclosed, double-digit tiered royalties on ex-US sales. In Dec 2021, Janssen terminated the agreement with Theravance after the failed performance of TD-1473.
.png)
Atea Pharmaceuticals terminated its co-development and commercialization deal with Roche
In Oct 2020, Atea granted Roche rights to co-develop and commercialize AT-511, AT-527, and their backup compounds (including AT-752) as therapeutics and companion diagnostics for infectious diseases except for Hep C. AT-527, an investigational oral direct-acting antiviral drug was being developed for the treatment of COVID-19. Roche had exclusive rights for the compound and diagnostics ex-US and non-exclusive rights in the US. Atea would be responsible for the manufacturing of AT-527. In addition, both the companies would jointly develop specific programs, including AT-527, on a global basis for COVID-19. Upon regulatory approval, Atea was responsible for the commercialization activities in the US and had a one-time option to request that Roche co-promote. Roche was responsible for the commercialization activities ex-US In Feb 2021, Roche sublicensed the Japan rights of AT-527 to Chugai. Atea received $350M upfront in Nov 2020 and was eligible to receive up to $330M in development and regulatory milestones and up to $320M in sales milestones, plus low double-digit to mid-twenties royalties. In Oct 2021, Roche terminated the agreement, followed by a P-II setback.
.png)
Mesoblast terminated its development and commercialization deal with Novartis
In Nov 2020, Mesoblast granted Novartis exclusive, worldwide rights to develop and commercialize remestemcel-L cell therapy to treat acute respiratory distress syndrome (ARDS) associated with COVID-19. Novartis agreed to provide support for commercial manufacturing scale-up and had the option to distribute remestemcel-L for graft versus host disease (GVHD) outside Japan. Additionally, both companies had the rights to co-fund development and commercialization for other non-respiratory indications, and the profit was to be shared equally. Mesoblast received a $50M upfront, including $25M equity. In addition, it was eligible to receive up to $505M in pre-commercialization milestones for ARDS indications and up to $750M in sales-based milestones, plus royalties. In Dec 2021, Novartis terminated the agreement with Mesoblast to develop and commercialize remestemcel-L cell therapy as the trial failed to meet the primary endpoints of mortality reduction.
.png)
BeiGene terminated its development and commercialization deal with Celgene
In Jul 2017, BeiGene granted Celgene exclusive, worldwide ex-Asia rights to develop and commercialize its PD-1 inhibitor, BGB-A317 (tislelizumab), for solid tumors. BeiGene retained exclusive rights to BGB-A317 for hematological malignancies worldwide and solid tumors in Asia ex-Japan. BeiGene purchased Celgene's commercial operations in China and gained an exclusive license to be responsible for commercializing Celgene's approved products in China: Abraxane, Revlimid, and Vidaza. BeiGene also received rights to commercialize CC-122 in China under the same terms as the approved products. BeiGene received $263M in upfront and $150M in equity by purchasing 32.7M, or 5.9 percent, of BeiGene's ordinary shares at $4.58 per share, or $59.55 per BeiGene American Depositary Shares (ADS). In addition, BeiGene is eligible to receive up to $980M in development, regulatory, and sales milestones for BGB-A317, plus undisclosed royalties. In Jan 2019, BMS' acquisition of Celgene mandated that BMS not hold two PD-1 assets. Further- Celgene decided to terminate its agreement with BeiGene for BGB-A317 and paid $150M as an exit fee. In Mar 2020, China NMPA suspended the importation, sales, and use of Abraxane in China and BMS terminated the deal associated with Abraxane, followed by a 180 days notice period. In Oct 2021, Celgene terminated the agreement related to Abraxane. The companies continued the commercialization of Revlimid and Vidaza.
.png)
Argenx terminated its development and commercialization deal with Cilag
In Dec 2018, Argenx granted Cilag (an affiliate of Johnson & Johnson), exclusive, worldwide rights to develop and commercialize Cusatuzumab (ARGX-110) for the treatment of acute myeloid leukemia (AML), high-risk myelodysplastic syndrome (MDS), and other hematological malignancies. Argenx has the option to co-promote the product in the US Argenx received $300M upfront, $200M in upfront equity from Johnson & Johnson Innovation – JJDC representing 4.68% of argenx's shares for $122.44 per share and was also eligible for up to $1.3B in milestone payments. Cilag would share economics 50/50 on a royalty basis in the US and double-digit royalties outside the US. In Jun 2021, Cilag terminated the agreement with argenx following Janssen's review of all available Cusatuzumab data and considering the evolving SOC for the treatment of AML.
.png)
Precision BioSciences terminated its Option and License Deal with Baxalta
In Feb 2016, Precision granted Baxalta an option to license exclusive, worldwide rights to CAR-T cell therapies for up to 6 undisclosed targets for cancer treatment. Precision was responsible for research activities until P-II, after which Baxalta had the right to opt-in for P-III development and commercialization. Additionally, Precision has the option to co-develop and co-promote the products in the US on a 50:50 basis. Precision received $105M upfront and was eligible to receive up to $1.6B in development, clinical, regulatory, and sales-based milestones, including an option fee, plus royalties. The agreement with Baxalta, which became the Shire, was assigned to Servier when Servier purchased Shire's oncology business in Aug 2018. In Sep 2020, Precision and Servier expanded the agreement to include two additional hematological cancer targets and two solid tumor targets. In Apr 2021, Precision and Servier mutually terminated the deal as both the companies entered into a Program Purchase Agreement.
.png)
Pfizer terminated its development and commercialization deal with Eli Lilly
In Oct 2013, Pfizer granted Eli Lilly worldwide rights to develop and commercialize tanezumab to treat osteoarthritis pain, chronic low back pain, and cancer pain. Pfizer received $200M upfront and was eligible to receive up to $350M in regulatory milestones and up to $1.2B in sales milestones. Both the companies would split the cost and profit equally. In Oct 2021, Eli Lilly and Pfizer discontinued the development of tanezumab following the negative feedback from both US and European regulators.
.png)
Voyager terminated its development and commercialization deal with Neurocrine
In Jan 2019, Voyager granted Neurocrine rights to develop and commercialize its gene therapy program NBIb-1817 (VY-AADC) for Parkinson's disease, VY-FXN01 for Friedreich's ataxia, and two undisclosed discovery programs. Voyager received $115M upfront in cash and $50M in equity investment at $11.9625 per share and was eligible to receive up to $1.7B in development, regulatory, and commercial milestone payments for four programs. Neurocrine funded the P-II/III pivotal study of NBIb-1817. Voyager had an option to co-commercialize the drug in the US following the data readout of the P-II RESTORE-1 trial. The companies would share costs and profits in the US equally and royalties in the rest of the world. Voyager was eligible for milestone payments, plus royalties based on global sales if not exercised its option. For VY-FXN01, Voyager was eligible to receive undisclosed research funding for P-I and had an option to co-commercialize VY-FXN01 for a 60/40 profit split in the US. Sanofi Genzyme holds the option rights in the rest of the world. Voyager received undisclosed research funding for two undisclosed drugs and was also eligible to receive undisclosed milestones, plus royalties. In Feb 2021, Neurocrine terminated the Parkinson's disease collaboration with Voyager following the roadblock of P-II candidate NBIb-1817 and clinical hold by the Food and Drug Administration.
.png)
Sosei Heptares terminated its development and commercialization deal with Allergan
In Apr 2016, Heptares granted Allergen exclusive, worldwide rights to develop and commercialize its portfolio of subtype-selective muscarinic receptor agonists to treat neurological disorders, including Alzheimer's disease. The agreement covers a portfolio of small molecule agonists targeting muscarinic M1 and M4, including P-I candidates HTL-9936 and HTL-18318 and dual M1/M4 receptors discovered using Heptares' proprietary StaR technology platform. Heptares will be accounted for the development of the program until the P-II study. Allergan was responsible for all further development and commercialization. Heptares received $125M upfront and was eligible for up to $665M in development and regulatory milestones (subjected to the clinical achievement and the launch for the first three licensed compounds) and up to $2.5B in sales-based milestones, plus double-digit tiered royalties. In addition, Heptares was also eligible to receive $50M for a research program that was to be conducted jointly by both companies. In Jan 2021, Sosei terminated the partnership based on business decisions regarding AbbVie's pipeline strategy due to its acquisition of Allergan.
.png)
Merck terminated its development and commercialization deal with GSK
In Feb 2019, Merck granted worldwide rights to GSK for the co-development and commercialization of M7824 (bintrafusp alfa), a bifunctional fusion protein immunotherapy for the treatment of non-small cell lung cancer (NSCLC). Merck received $341M upfront and was eligible to receive up to $569M in development milestones and up to $3.3B in regulatory and commercial milestones. Both companies would split the cost and profit equally globally. In Jan 2021, an independent monitoring committee reported that M-7824 would not meet its primary endpoint. On 16 Mar 2021, the independent review showed an objective response rate of 10.1%, with efficacy hitting below a predefined threshold. In Sep 2021, Merck and GSK terminated the agreement.
.png)
Galapagos terminated its development and commercialization deal with Gilead for GLPG-1690
In Jul 2019, Galapagos granted Gilead exclusive rights to develop and commercialize its P-III drug candidate, GLPG-1690, for idiopathic pulmonary fibrosis plus an exclusive option to license the US rights to GLPG-1972 after the completion of an ongoing P-IIb study for osteoarthritis. Both companies signed a ten-year research partnership to develop six clinical molecules, more than 20 preclinical molecules, and a discovery platform. Galapagos was accountable for the research until the completion of the P-II study. Gilead had an option to license the program excluding Europe if exercised. Galapagos and Gilead were equally responsible for the development cost. Galapagos had a right to expand the collaboration three years for those programs entering clinical development before the end of the partnership. Galapagos received $3.95B upfront and $1.1B in equity investment at EUR 140.59 per share at a 30-day premium of 20%, bringing Gilead's stake from 12.3% to 22%. Gilead received warrants to acquire 2.6M shares of Galapagos ordinary share subject to a ten-year standstill restricting Gilead from increasing the stake beyond 29.9%. Galapagos was eligible to receive up to $325M in milestone payment based on the GLPG-1690 approval in the US. Galapagos to receive $250M opt-in payment and up to $200M for secondary efficacy points and was also eligible to receive up to $550M in regulatory and commercial milestones. Galapagos was eligible to receive $150M as an opt-in payment per program but not for the milestone payments for the research programs. Galapagos received tiered royalties ranging from 20-24% for all licensed programs. In Feb 2021, Galapagos and Gilead terminated the deal after the halt of ISABELA P-III clinical studies with GLPG-1690. The companies will continue the development of its option products, including GLPG-1972.
.png)
SOBI terminated its agreement with Advent International
In Sep 2021, Advent International and GIC Special Investments signed a deal to acquire SOBI, a rare disease drug maker. SOBI would receive $8B at $27.49 per share at a one-day premium of 34.5%. In Dec 2021, SOBI terminated the agreement with Advent as certain stakeholders, including AstraZeneca, who had an 8% stake, withheld its buyout offer.
Related Post: Top 20 Biopharma Deal Terminations of 2020 Based on Total Deal Value
Tags

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com