Exclusive Coverage: Key Takeaways from the 6th Annual Medical Device Regulatory Quality Summit 2022
India is among the top 20 markets for medical devices worldwide. India's medical devices market stood at $11Bn in 2020 and is expected to reach $65Bn by 2024. The Government of India (GOI) has commenced various initiatives to strengthen the medical devices sector, with emphasis on R&D and 100% FDI for medical devices to boost the market.
The 6th Annual Medical Device Regulatory Quality Summit 2022 held on 12-13th October 2022 conceptualized by Inventicon Business Intelligence in New Delhi, India in collaboration with Elmas Medical as gold partners and Starlims as silver partners. New upcoming medical device regulations, Quality management in view of new regulations and patient safety, and the evolution of the latest technologies were the highlights of the two days session. Find out the key takeaways from this medical device conference.
Day 1:
The day 1 session started with a panel discussion. The session was on the Indian Medtech Regulatory and Policy Framework: Ready to harness global opportunities

The session was led by Vibhav Garg, Director-Health Economics & Govt Affairs, India HUB & ASEAN, Boston Scientific
The first panelist for the day was Urvashi Prasad, Director, Development Monitoring & Evaluation Office, NITI Aayog
Urvashi Prasad talked about strengthening the overall regulatory policy. She said that having a conference like this on the medical devices bill and the policy, what are some of the regulatory policies that we need to change we need to bring. She highlighted the Think Tank policy of the Indian Government and how Niti Ayog is working on the different aspects of strengthening the medical device sector and promoting Innovation in medical devices.
“So, we have been thinking about how we can offer Research linked Incentives to medical device manufacturers and innovators so that we can catalyze more innovation in this sector. We have been also looking at how we can streamline the regulatory processes to make it easier for manufacturers to get approval, to get the license so that is another aspect that Niti Aayog has been working on just, in general, strengthening the overall regulatory policy and framework in the country”
The second panelist for the day was Rajiv Nath, Managing Director of Hindustan Syringes & Medical Devices Ltd. Founder and Forum Coordinator – Association of Indian Medical Device Industry (AiMeD)
Mr. Rajeev Nath specifically emphasized on the risk proportionate rulebook for manufacturers. He highlighted that the 3rd area was off regarding the issue of low-risk classic devices like in the USA & Canada for example. The no measurable, non- sterile medical devices like WheelChairs, Spectacular Frames don't require stringent systems which are required like syringes.
“So, for that, we have sought some similar assumptions so we don`t need to make them so strict for these manufacturers which are single women/men entrepreneurs who manage companies that don't have systems and places but still make quality products by their supervision”
“He also said that the Indian Exports last year crossed more than 44000 crores we are growing more than 15% per annum towards exports. So, the current ratio in the Indian market must be higher than in the global market. My company alone exports to more than 200 countries in the world during the covid time we supplied syringes to Japan and Brazil.”
The third panelist for the day was Mr. PV Mathew, Sc-E and Head (MHD) Bureau of Indian Standards
PV Mathew highlighted the importance of standardization of medical device industries and whether we can make it globally competitive.
The event continued with a presentation from Mr. Sudhakar Mairpadi, Director- Regulatory & Government Affairs, Philips India Ltd. on “Understanding the benefits of harmonized standards for medical devices”. The presentation highlighted:
- Strategies for developing a unified QMS that governs all quality systems from R&D through to final product testing
- Understanding the global approach to a single audit program
- Single audit program that satisfies the relevant requirements of the regulatory authority, and enabling faster market access

The second panel discussion was led by Dr. Gridhar J. Gyani, Director General, Association of Healthcare Providers- AHPI India
The panelist for the session were:
Lipi Chakhaiyar, Associate Director - Regulatory Affairs, Abbott Healthcare Pvt. Ltd.
Sudhakar Mairpadi, Director- Regulatory &Government Affairs, Philips India Ltd.
Alok Kumar Soni, Head Regulatory Affairs & Quality Assurance- India & South Asia, Bio-Rad Laboratories (India) Pvt. Ltd.
The key highlights of the panel discussion were:
• The status of preparation and implementation
• The impact of implementing and delegated acts on notified bodies
• Clarifying expectations for how the industry should be prepared and implementing
Dr. Giridhar J. Gyani highlighted two most important and best initiatives by the GOI Ayushman Bharat and National Digital Health Vision
“These are the two most important and best initiatives by the Government of India 40% of the government of India is provided with free healthcare coverage and similarly the National Digital Health mission is important because keeping in view the shortage of doctors and numbers of bed the technology will be the great facilitator in reaching out to the masses in the rural area and at work, I keep saying that if these two initiatives succeed in next two years India”
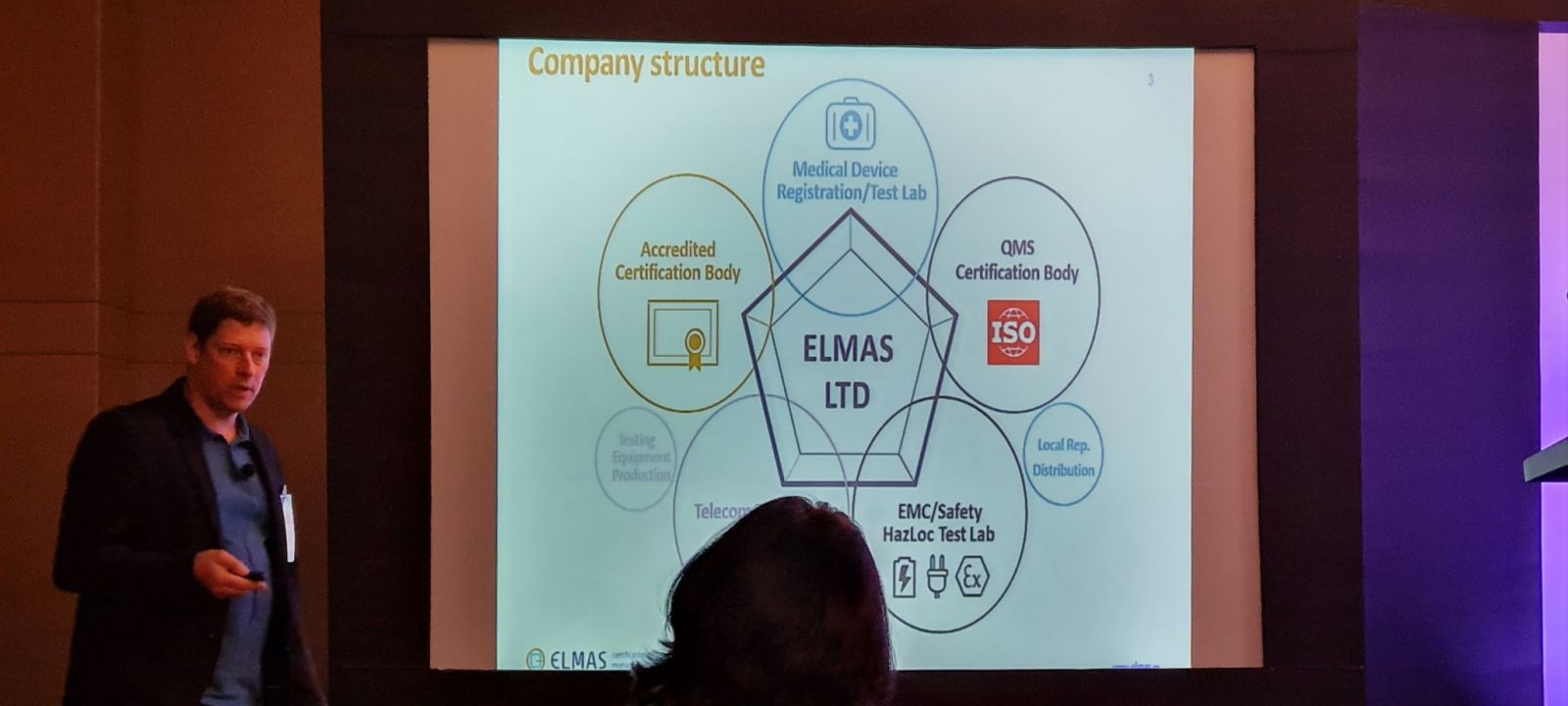
The panel discussion was followed by a presentation from Andrey Fadeev, CEO, of Certification and Regulatory Affairs Russia & EAEU Elmas-Medical on “Medical Device registrations in Russia”
Andrey Fadeev highlighted the regulatory consulting in Russia, the US, and Union Countries for medical device projects.
The next presentation was by Dr. Ashish Indani, Senior Manager of Clinical Affairs, Stryker Global Technology Center on “Changing needs for clinical evidence with changing global regulations”. The presentation highlighted:
- Clinical Evaluation Report requirements for various countries GSPRs vs. EPs
- Clinical Evidence Report requirements for Japan, the US, and other countries
- Accepted sources of clinical data and their evidence level
- How much data is sufficient?
The series of presentations was continued by Shivkumar Vishwanathan, B.E. (Chem), Dip Packaging Technology Director, Poly-Med Analytical Pvt. Ltd. on the “Chemical Characterization of Medical Devices as per ISO 10993-18”. The presentation highlighted:
- Supporting biological safety
- An important but less understood tool
- A pathway to optimizing testing
To continue the series “Accreditation: Supporting Quality of Medical Devices & Regulatory Compliance” was presented by Varsha Mishra, Deputy Director, NABCB, National Accreditation Board for Certification Bodies Quality Council of India
The presentation titled as “Regulatory approvals for Software and artificial intelligence based medical device under EU MDR” was presented by Sundeep Agarwal, General Manager-Compliance & Regulatory Affairs, Datt Mediproducts
The key highlights of the presentation were:
- Learning the correct software classification’
- Software verification and validation
- Technical Documentation for SaMD and AI devices
- Core factors that will achieve regulatory approvals
He said : “The resource content of this program has really increased and it has a lot of insights that are required irrespective of your devices and domain. You`ll have a lot of information right from the I would say, Team leaders or Leaders of the Industry that’s very informative. Secondly, you need to enhance your knowledge and this is one way of doing this if you ever had a busy schedule. It's really a get-together for many of the industry experts who are looking for a lot of information. What you don`t know you get to know and what you want to share then you just have to go ahead. So, it brings everybody on the same platform and talks together so collectively have 100-200 years of experience”
The event continued with the third-panel discussion led by Parveen Jain, Head of Regulatory Affairs, Quality and Government Affairs, Fresenius Medical Care (India) Pvt Ltd. on “Evolving device regulations- ensuring compliance, quality, and patient safety”
The panelists for the session were:
Mr. Madhusudhan D H, Site Quality leader, RCS Quality, Medtronic India Development Center
Mrs. Mamatha S G, Executive Global Quality Systems & Total Quality India Leader India Sites, GEHC-SA I&D Leader, GE Healthcare
Mr. Sahjogita Kathuria, Director of Regulatory Affairs & Quality Assurance, Terumo India Pvt. Ltd.
The key highlights of the session included:
- Essential principles of safety and effectiveness for medical
- devices and the role of standards
- Tools and strategies for quality improvement and patient safety
- Measures and benchmarks to achieve desired outcomes
The Day 1 Ended with hope to look forward to Day 2 events
The Day 2 session was started by Mrs. Mamatha S G, Executive Global Quality Systems & Total Quality India Leader India Sites, GEHC-Sa I&D Leader, GE Healthcare on “Medical device design controls: Why, When, What and How?”
The highlights of the session were: -
- Documenting design procedure, development, and planning
- Conducting risk analysis and design reviews
- Executing design verification and validation (software and/or hardware)
- Controlling design changes and reviewing design results
- Transferring design to manufacturing
Mrs. Mamatha S G highlighted the medical device design controls.
“Medical devices are the big growth market in India because it is an untouched area pretty much so you will see a lot of MedTech companies coming up and also existing big MedTech companies are coming up with different product lines to touch the area we don't have the healthcare provided as if now so it’s a huge market.”
“The trust in the medical devices pros and the medical approval work. The trust comes up with the trust in the regulations. I say today my product is approved or I have a CE mark on the product then people will buy the certification or the approvals that the regulators will give for the medical devices will build trust in us. While the covid lot of innovations come up, we don't have much trust in the product because people couldn’t relate it to say if there is a good clinical evolution done on this product because short-term products people will start dogging are effective or not I think if we can give. If people start trusting regulators then their trust will automatically come to the medical devices also”
The series of presentation was continued by Madhusudhan D H, Site Quality Leader, RCS Quality, Medtronic India Development Center on “Preparing for inspections to meet increasing Quality and regulatory Demands”
The key takeaways of the session included: -
- Best practices for preparing manufacturing partners and suppliers
- Ensuring company readiness for positive inspection outcomes
- Acknowledging published records to ensure corrective action
The next insightful session was by Bivash Chakraborty, Head- Regulatory Affairs & Quality & Public Business- India & south Asia, Biomerieux India Pvt. Ltd. on “Reviewing the IVD registration requirements and expectations considering the Drugs Medical Device and Cosmetics Bill 2022”
The session highlighted:
- Outlining the classification of IVDs across India: Medical device, Pharmaceutical or other?
- Examining the practical implication or the new device law on IVDs
- Outlining points of contact and sources of information for IVD market registrations
Bivash Chakroborty highlighted that the regulations for medical devices and IBDs are getting revised from time to time and medical devices & IBD are one important sector for the Government of India. There are a lot of new initiatives taken by the Government.
He said that “medical devices are now currently 80% imported which the importance of Initiatives by the Government like to reduce to 60% in the next five years and finally to 40% imported and 60% manufactured by 2030. So, these are the initiatives where a lot of manufacturers will start manufacturing the products which can be manufactured in India and less imported and this will also give opportunities to a lot of Multinational Companies to start manufacturing in India”
“I think this is the right time for medical devices and Input Diagnostics. And the market is also growing in Double digits and there is a lot of focus on the post-Covid on Health and Primary Healthcare so there is a general awareness of Health and that also gives a lot of medical tourism in India and a lot of medical patients are coming into India for the treatment and the 1/10th of the cost so India has got a lot of focus for the Medical Devices, Medical Tourism and even for the treatment”
The series was continued by Preety Sharma, Head- Regulatory Affairs, Edwards Lifesciences on “Rules for Labels & Information provided with medical devices”
The key takeaways of the session were:
- E-labelling: Review of new regulations & requirements
- Streamlining accompanying information with global standards
- Further learning for fully compliant labeling
The second day continues with the first panel discussion of the day led by Sumati Randeo, Director- Government Affairs, DHR Holding India Pvt. Ltd. on “Understanding the EU MDR & IVDR-reaching to a consensus in transition timeline interpretations on Medical Devices and IDVs”
The panelist for the session were:
Preety Sharma,Head- Regulatory Affairs, Edwards Lifesciences
Prashanth Prabhakar, Head- Regulatory Affairs, Boston Scientific India Pvt. Ltd.
Bisvash Chakraborty, Head- Regulatory Affairs & Quality & Public Business- India & South Asia, Biomerieux India Pvt. Ltd.
Krishnamurthy Thyagarajan, Senior Manager- Quality and Regulatory Affairs,Beckman Coulter India Pvt. Ltd.

The session summarized the following key points:
- Common misinterpretations across the industry
- Review of exceptions to the EU MDR and IVDR transition timelines
- Medical Device Directive product certificates that go beyond 2022
Day 2 continued with a virtual session by Dr. Ashok Thakkar, Head- Clinical Research, Meril Life Sciences Pvt. Ltd. on “How to conduct a successful trial?”
The next in line was the presentation by Mr.Hari Babu Sudharsanam, Research Scientist I- Biocompatibility Lead Preclinical QA India, Baxter Innovations and Business Solutions Pvt. Ltd. on “Current challenges in Biocompatibility testing of Medical devices”
The presentation highlighted:
- Overview of Biocompatibility
- Current challenges in test methods
- Ways to mitigate the risk
Day 2 ended with an Open House Discussion: Lessons Learned in achieving MDR certification which included an open Q&A session between all the delegates of the conference. It was insightful and a positive encouragement for people in the healthcare and medical device industries.
End of conference
Related Post: Exclusive Coverage: Key Takeaways from the DIA India Medical Devices Conference 2020
Tags

Senior Editor at PharmaShots. She is curious and very passionate about recent updates and developments in the life sciences industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots.