Disease of the Month: Age-Related Macular Degeneration
Introduction:
AMD, an eye disease that can cause of loss of eyesight/blur vision mostly in people ≥50 years of age and it is the leading cause of visual impairment worldwide.
It occurs when the part of the retina (macula) is damaged. Patients lose their central vision and cannot see fine details
The three stages of AMD are Early, Intermediate, and Late stage
February is known as AMD awareness month and low vision awareness month
The two types of AMD are:
1: Dry AMD- It is also called atrophic AMD. It occurs when macula gets thinner with age. It usually progresses slowly over several years. There’s no treatment for late dry AMD
2: Wet AMD- Also called advanced neovascular AMD. It occurs when abnormal blood vessels grow in the back of the eye and damage the macula. Dry AMD can turn into wet AMD but wet AMD is always late stage. It is the more advanced and damaging form of AMD
Some patients with AMD will develop geographic atrophy (an advanced and severe form of dAMD) that can lead to permanent vision loss and not any approved therapies are available to treat geographic atrophy
Risk Factors:
Ageing: People with age group ≥50 is prone to develop AMD
Race: It more often affects whites than African Americans and Hispanics
Blood Pressure: Person with high cholesterol, and high blood pressure are more prone to develop AMD
Genetic: People with a family history of AMD are at greater risk to develop AMD
Smoking: Studies show that smokers and ex-smokers are more likely to get AMD than people who never smoked
Symptoms:
Symptoms of AMD include:
- Blurry or fuzzy vision
- Straight lines appear wavy
- A dark, empty area or blind spot appears in the centre of vision
- Loss of central vision, which is necessary for driving, reading, recognizing faces and performing close-up work
- Difficulty recognizing familiar faces
Diagnosis:
The two ways to diagnose AMD are:
- Comprehensive Dilated Eye Exam: In this process an ophthalmologist gives some eye drops to dilate (widen) the pupil and then check for AMD. It includes various tests such as, visual acuity test (for clarity), visual field test (for peripheral vision), tonometry test (pressure in eyes), pupil response test (way of lights enter into eyes)
- Optical Coherence Tomography: A non-invasive retina imaging test that uses light waves to take high resolution cross-section pictures of retina.
- Fluorescein angiography: Used to detect wet AMD, a special dye injected into a vein in the arm and as the dye passes through the blood vessels in the retina, pictures are then taken and evaluated by the doctor if the blood vessels are leaking and whether or not the leaking can be treated
- Amsler grid: This one is also for wet AMD; this test uses a checkerboard like grid to determine if the straight lines in the pattern appear wavy or missing to the patient
- The ForeseeHome Monitoring Device, a device to monitor dry AMD at home. It comes under Medicare-covered service for patients enrolled in Medicare across the US, and who are at high risk for converting dry AMD to wet AMD. It detects changes before a patient would notice within ~3 mins. Eye and allows the doctor to monitor the vision for any changes that take place between regularly scheduled exams
Epidemiology:
According to the Association for Research in Vision and Ophthalmology (ARVO), prevalence of AMD is estimated at ~196M affected individuals and expected to rise up to ~288M by 2040
of them ~2M effected population with visual loss are in the US and expected to rise ~5M
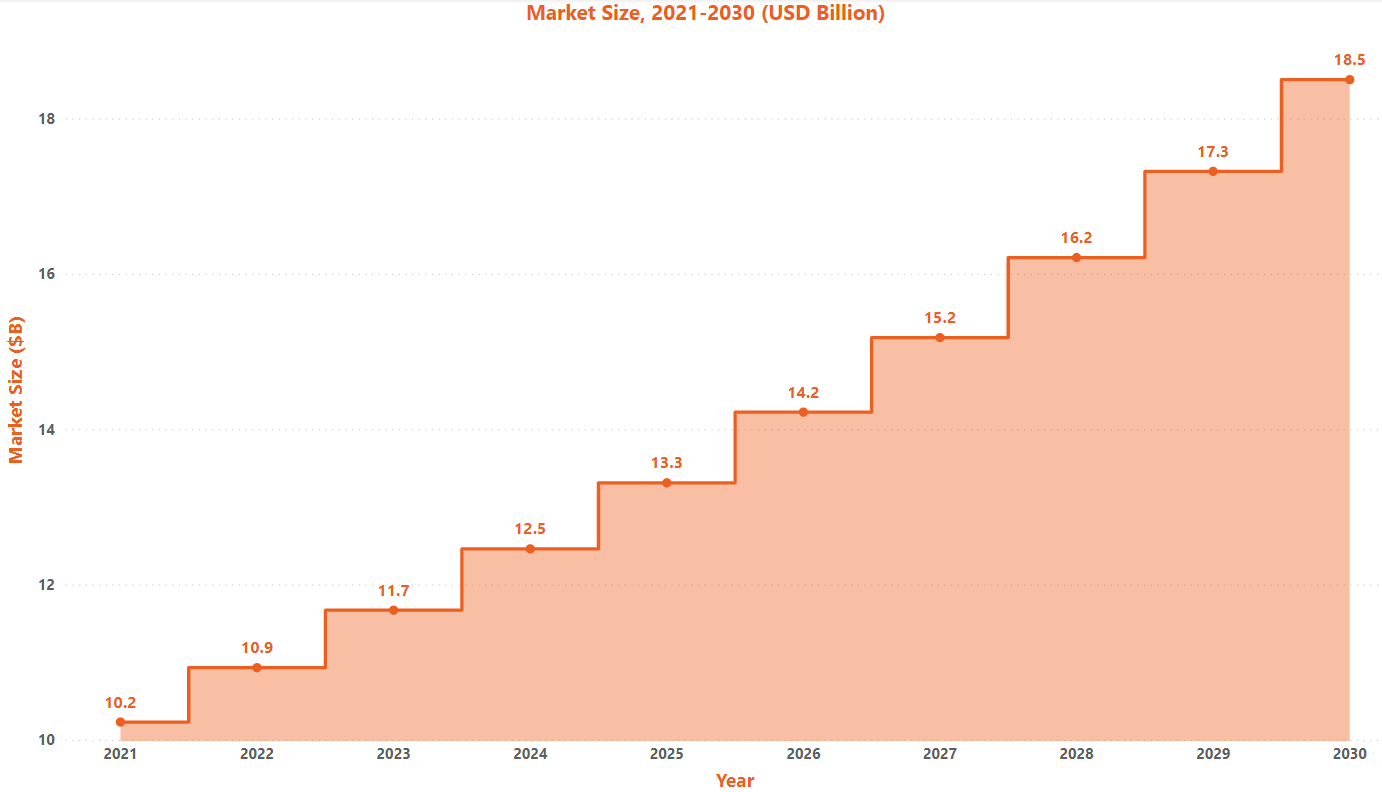
Market Size:
The global AMD market was at $10.23M in 2021, and at CAGR of 6.8%, it is expected to reach $18.5M by 2030
Treatment:
AMD treatment depends on the stage and type, currently no treatments options are available for early AMD. All the available options can only pause it from progression into late AMD or vision loss
- Anti-angiogenic drugs/Anti-VEGF drugs: They stop new blood vessels from forming and stop the leaking from the abnormal vessels that cause wet AMD. Some of the approved therapies are:
- Vabysmo (Faricimab), VEGF/Ang-2 inh., marketed by Roche and available at the initial dosing of 6mg, intravitreal injection, Q4W (approx. Q4W ± 7 day, monthly) for the first 4 doses are recommended
- Eyela (Aflibercept), VEGF inh., marketed by Regeneron in the US, the EU, Canada, Japan and China at initial dosing of 2 mg every 4 wks. x 3 injections
- Photodynamic therapy (PDT): Doctor injects light-sensitive drug into bloodstream to damage abnormal blood vessels followed by this laser treatment will be used to activate the drug. It creates blood clots in the abnormal blood vessels to stop them from leaking
- Laser therapy. A treatment with high-energy laser light that can seal and sometimes destroy actively growing abnormal blood vessels from wet AMD
- AREDS 2 Supplements: A special dietary supplement and an updated version of AREDS (it included Beta-carotene) that can help slow down vision loss for intermediate AMD. It is a combination of large amounts of vitamins and minerals incl. Vitamin C (500mg), Vitamin E (400IU), Copper (2mg), Zinc (80mg), Lutein (10mg), Zeaxanthin (2mg) but excluded Beta-carotene
- No approved therapies are available for the late dry AMD
Key Players in the Market:
Top Key players in the global market with approved drugs for the treatment of AMD includes Regeneron, Genetech, Roche, Novartis etc.

*The above-mentioned table include some of drugs approved in the US and the EU
Clinical Trial Analysis:
Anti-VEGF receptor antagonist, Protein kinase C inhibitor, Autotaxin inhibitor etc are some of the MoA based on that molecules are in development to treat AMD that inhibits the growth of new blood vessels to prevent leakage.
Key players in this space are Boehringer Ingelheim (BI-836880), Clearside Biomedical (CLS-AX), Kodiak Sciences (SI-301) Ashvattha Therapeutics (D-4517.2)
All the current SoC having limitations of frequent administration of anti-VEGF injection into the eye. Thus, to overcome these limitations, various researches are ongoing to develop gene therapies as single administration for wet AMD to decrease the limitation of conventional therapies
- REGENXBIO, is one of the leading companies which is currently enrolling participants to evaluate gene therapy, RGX-314 in clinical studies (ATMOSPHERE and AAVIATE) for subretinal and suprachoroidal delivery for wet AMD
- NEI (National Eye Institute) research team has developed a new stem cells-based treatment for dry AMD and safety investigation is ongoing
Based on G10 geography distribution, the interventional clinical trials are classified in the below mentioned graph in two groups based on its status i.e., active (recruiting, active, not recruiting, not yet recruiting and enrolling by invitation) and inactive (withdrawn, suspended terminated and trials with unknow status). The interpretation showed that highest no. of trials being conducted in the USA and the least number of trials are reported in India (as represented in the graph)


*This graphical interpretation is based on our point of view of the clinical data
Government Benefits:
Federal, state, and local government agencies provide free or low-cost services to the people having difficulty paying for eye care services or medical treatment that includes:
- Medicare: This is a federal health insurance program for people ≥65 who are receiving Social Security retirement benefits also provides prescription drug coverage
- Medicaid: This program is administered by state agencies to economical weak people
- US Department of Veterans Affairs: VA provides access and coverage for eye care services to eligible veterans
Financial Aid for Macular Degeneration Medications
Some of the organizations are available which cover the costs of macular degeneration prescription medications via various programs such as:
- BlinkHealth, GoodRx, Medicare (Prescription Drug Discounts/Plans) ConnectiveRx (Aerie Savings Card)
- RxHope, Rx Outreach, Pan Foundation, NeedyMeds, Eagle Pharmacy (Patient Assistance Programs)
- ‘In My Eyes app’ is a virtual platform developed by Regeneron that allows viewers to experience the blurriness, wavy lines or black patches caused by different types of retinal disease. Along with this Regeneron provides Eylea Patient Support Program incl. Eyela Co-pay Card Program
- BrightFocus Foundation also funds some scientific research worldwide based on macular degeneration
References:
For Deep dive landscape please mail us at connect@pharmshots.com



