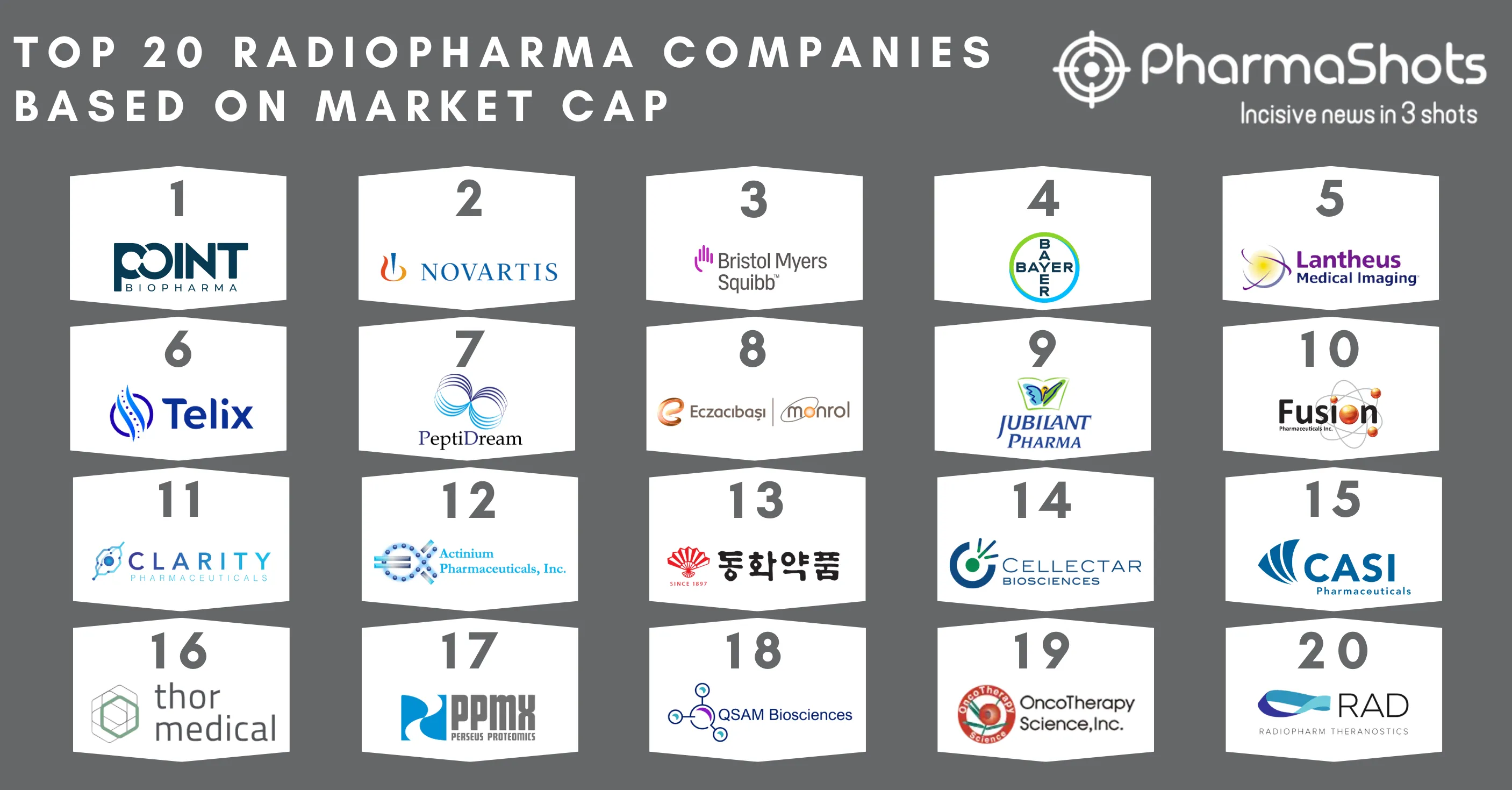
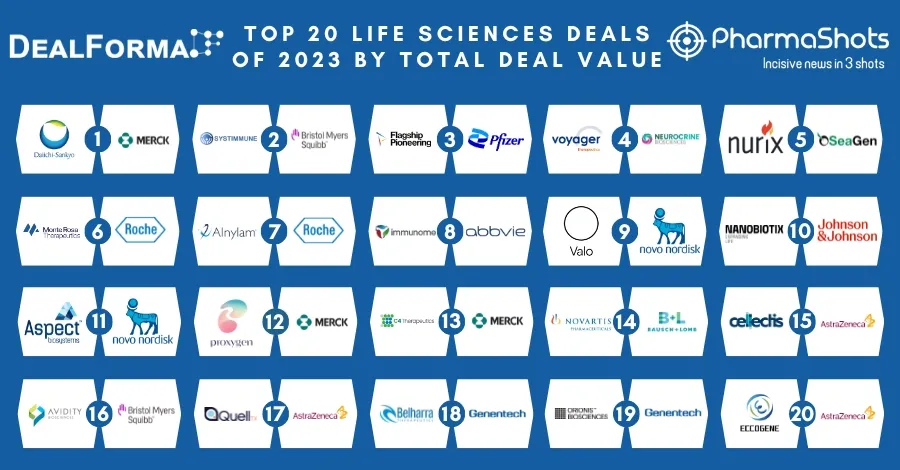
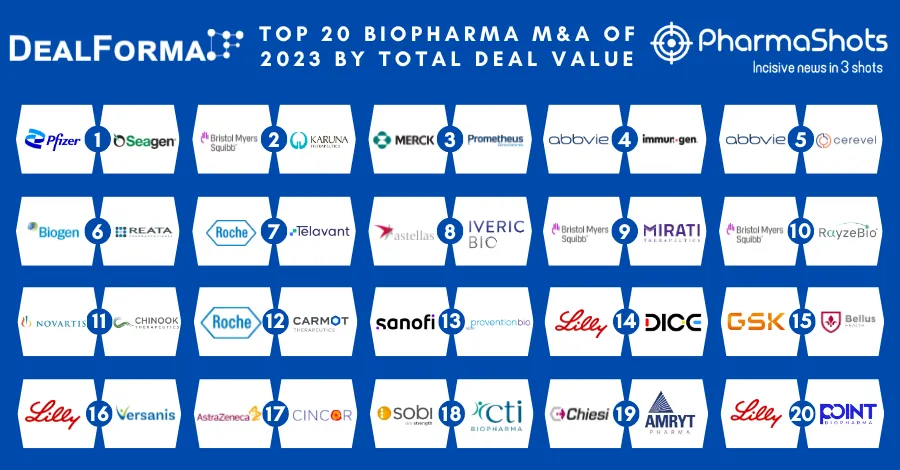
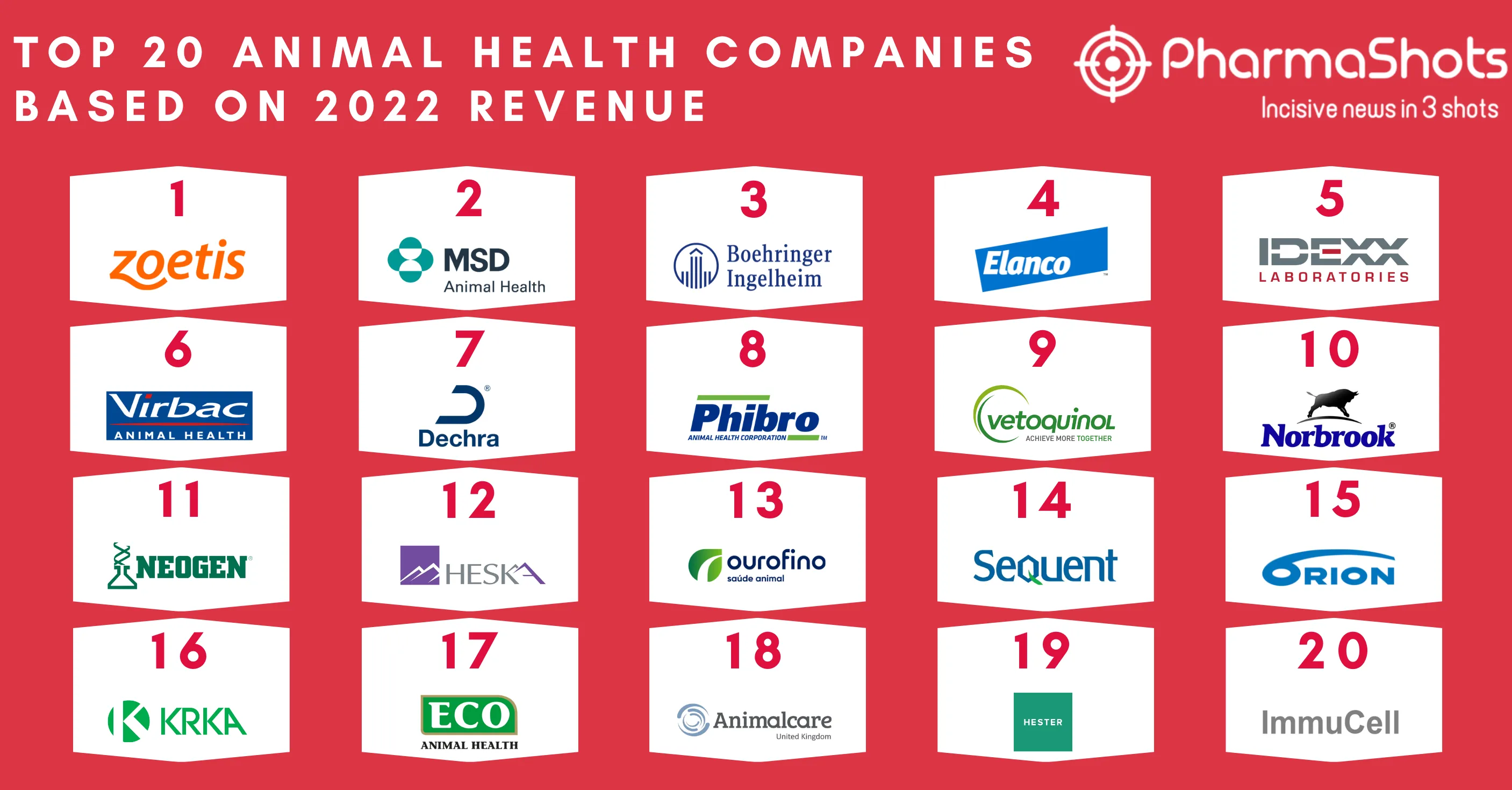
Top 20 Drugs with US Patent Expiry in 2023 Based on Total Sales Value
Shots:
- Drug patent expiry is when a patent granted to a pharmaceutical company for a particular drug expires, allowing other companies to produce and sell generic versions
- Like every other utility patent, pharmaceuticals also get market exclusivity of 20 years. Some companies accept the offered period and open doors to biological drugs or biosimilars. In contrast, others try different strategies like new formulations, developing alternative administration techniques, and modifying the drug uses to extend the market exclusivity
- While several other factors, like the unanticipated arrival of a better drug, failure to disburse maintenance fees, and post-M&A restructured pipelines, can be early instigators of patent expiry. PharmaShots has prepared a list of the top 20 drugs with patent expiry in 2023

Company: Eisai
Generic Name: Rufinamide
First Approval Date: Nov 17, 2008
Patent Expiration: Apr 2023
2022 Sales: $52M
Indication: Lennox-Gastaut syndrome (LGS)
Banzel is a prescription add-on drug for the treatment of seizures linked with LGS. Banzel works for around ten different types of seizures by reducing their frequency and severity. The exact mechanism of action is uncertain. But it's believed that rufinamide modifies sodium channel activity, particularly by prolonging the channel's inactive state. Lupin has earned the US FDA approval for the generic version of Banzel as Rufinamide Oral Suspension. Glenmark has also released its generic drug version in the US market.

Company: AVEO Oncology
Generic Name: Tivozanib
First Approval Date: Mar 10, 2021
Patent Expiration: Apr, Nov 2023
2022 Sales: $110M
Indication: Renal Cell Carcinoma (RCC)
Fotivda is the first and only the US FDA approved drug for the treatment of advanced kidney cancer or RCC patients who have been treated with two or more prior therapy and have not responded to treatment or had a reoccurrence. The drug selectively inhibits the phosphorylation of VEGFR cells, c-kit and PDGFR-ß. As of now, there are no generic versions available for the drug at present.

Company: AstraZeneca
First Approval Date: Jul 31, 2009
Generic Name: Saxagliptin
Patent Expiration: Jan 2023
2022 Sales: $257M
Indication: Type 2 Diabetes Mellitus
Onglyza is a member of the dipeptidyl peptidase-4 inhibitor class of oral hypoglycemics used combined with diet and exercise for managing type 2 diabetes. It reduces blood sugar by prolonging the Glucagon-like Peptide-1 (GLP-1) and Glucose-dependent Insulinotropic Polypeptide (GIP) incretin activity in a glucose-dependent manner. So Onglyza reduces the sugar amount released by the liver overnight and increases insulin levels after meals to prevent after-meal blood sugar spikes. Currently, the drug is not accessible in generic form.

Company: Supernus Pharmaceuticals
Generic Name: Topiramate
First Approval Date: Aug 16, 2013
Patent Expiration: Jan 2023
2022 Sales: $261.2M
Indication: Epilepsy
Trokendi is an extended-release capsule used to treat epilepsy and prevent migraine headaches. The exact mechanism of action of the drug is unknown. Still, the drug is believed to increase GABA activity and inhibit glutaminergic receptors, thereby blocking overall neural hyperexcitability and preventing seizures and migraines. Zydus has developed and marketed the generic version of Trokendi in the US market.

Company: Sanofi
Generic Name: Plerixafor
First Approval Date: Dec 15, 2008
Patent Expiration: Jul 2023
2022 Sales: $281.5M
Indications: Non-Hodgkin’s Lymphoma (NHL) or Multiple Myeloma (MM)
Mozobil is an immunostimulant used in combination with Granulocyte-Colony Stimulating Factor (G-CSF) to mobilize hematopoietic stem cells in cancer patients into the peripheral bloodstream. The stem cells are then collected and subsequent autologous transplantation into the patients. It acts as a mobilization agent and should be injected subcutaneously with dosage depending on body weight. As of now, there are no generic versions of the drug available for now.

Company: Eisai
Generic Name: Eribulin Mesylate
First Approval Date: Nov 15, 2010
Patent Expiration: Jul 2023
2022 Sales: $297M
Indication: Breast Cancer and Advanced Liposarcoma
Halaven is a prescription drug for breast cancer patients with metastasis and liposarcoma who cannot be treated with surgery. It is the only drug in its class of chemotherapy and is developed from the natural substance found in a sea sponge. Halaven binds to the tumor, blocks cell division, and causes cell death. During 1st cycle of treatment, Halaven is injected as an infusion over a short period of 2-5 mins once a week for two weeks and skipped for one week. As of now, there is no therapeutically equivalent version of Halaven available.

Company: Novartis
Generic Name: Pazopanib
First Approval Date: Oct 9, 2009
Patent Expiration: Oct 2023
2022 Sales: $474M
Indications: Advanced Renal Cell Carcinoma (RCC), Advanced Soft Tissue Sarcoma (STS) with prior chemotherapy
Votrient is a targeted cancer kinase inhibitor with the active ingredient pazopanib. The drug blocks the protein kinases found in some receptors on the surface of VEGFR, PDGFR, and KIT – the cells involved in the growth and spread of cancer cells. It results in the inhibition of blood vessels formed in cancer cells, and eventually, the cells die. Votrient is available in the form of tablets of 200mg and is recommended orally once daily. For the time being, there is no generic version of Votrient available.

Company: Takeda
Generic Name: Dexlansoprazole
First Approval Date: Jan 30, 2009
Patent Expiration: Jan, Oct 2023
2022 Sales: $496M
Indication: Heartburn associated with symptomatic non-erosive gastroesophageal reflux disease
Dexilant is a proton pump inhibitor (PPI) that reduces the production of acid by the stomach. Dexilant works in two stages: first within an hour of administration and second after 4-5 hours from administration. Dexilant blocks acid production by specifically acting on the proton pump and suppressing the gastric acid secretion by inhibiting (H+, K+)-ATPase in gastric parietal cells. Last year TWI announced the US FDA approval of their generic Dexilant is available in 30mg and 60mg capsules which would be released into the market once the patents expire.

Company: Takeda
Generic Name: Teduglutide
First Approval Date: Dec 21, 2012
Patent Expiration: Mar 2023
2022 Sales: $540M
Indication: Short Bowel Syndrome (SBS)
Gattex is a subcutaneous injection used in SBS patients dependent on parenteral support. It is the first and only drug that acts like the naturally produced GLP-2. Gattex increases the villi height and depth between the villi resulting in increased absorption of fluid by the small intestine. The drug is prescribed for adults and children (≥ 1 yr). In the annual report of Takeda, the company reported the generic entry from the unnamed player after Mar 2023. However, according to the US FDA approval database, no generic drug maker can earn regulatory approval for their generics.

Company: Novartis
Generic Name: Amlodipine/Valsartan
First Approval Date: Jun 21, 2007
Patent Expiration: Apr 2023
2022 Sales: $743M
Indication: Hypertension
Exforge is a combination drug comprising two anti-hypertensive medicines: Amlodipine and Valsartan. Amlodipine acts as a Calcium Channel Blocker (CCB) and relaxes blood vessels. On the other hand, Valsartan is an Angiotensin II Receptor Blocker (ARB) blocks the interaction of angiotensin II with signaling proteins; the actions of both drugs result in a lowering of blood pressure. Sandoz, Teva, and Alembic are among the companies that have launched the generic versions of Exforge.

Company: Jazz Pharmaceuticals
Generic Name: Sodium Oxybate
First Approval Date: Jul 17, 2002
Patent Expiration: Jun 2023
2022 Sales: $1B
Indication: Narcolepsy
Xyrem is an oral solution for treating two common symptoms of narcolepsy, i.e., excessive daytime sleepiness (EDS) and cataplexy. It is unclear how the drug works, but experts think that when taken at night, Xyrem impacts the neurotransmitters (natural chemicals in the brain) to help relieve symptoms in patients. Hikma launched the generic Xyrem in the US in Jan 2023. Jazz Pharma has also inked a patent settlement with Amneal to launch a limited quantity of its generic after July 1, 2023.

Company: Novo Nordisk
Generic Name: Liraglutide
First Approval Date: Jan 25, 2010
Patent Expiration: 2023
2022 Sales: $1.7B
Indication: Type 2 Diabetes Mellitus
Victoza is a non-insulin, anti-diabetic medicine used for the treatment of type 2 diabetes and to reduce the risk of cardiovascular events associated with the disease. To control blood sugar levels, Victoza works in 3 ways (like GLP-1 hormone): it slows the exit of food from the stomach, it prevents the liver from producing too much sugar, and in case of high sugar levels, it supports the pancreas make more insulin by helping beta cells function normally. According to the Novo Nordisk legal settlement with its competitors, though the Victoza patent expires in 2023, the drug wouldn’t face generic competition until 2024. The companies awaiting their generic launch include Sandoz, Teva, and Viatris.

Company: Sanofi
Generic Name: Teriflunomide
First Approval Date: Sep 12, 2012
Patent Expiration: Mar 2023
2022 Sales: $2.2B
Indication: Multiple Sclerosis
Aubagio is a once-daily pill for treating relapsing forms of Multiple Sclerosis (MS). The drug is effective in treating the 2 sides of MS: firstly, it reduces inflammation (by decreasing relapses and the number of lesions), and secondly, it slows the disability progression. As the drug approaches its patent cliff, the generic companies are set to launch their Aubagio generics into the US market. In 2018, the US FDA granted ANDA approval to Glenmark's Teriflunomide Tablets in the strength of 7 mg and 14 mg. Teva is also ready with their Teriflunomide Tablets as an equivalent therapeutic version of Aubagio.

Company: AstraZeneca
Generic Name: Budesonide and Formoterol Fumarate Dihydrate
First Approval Date: Jul 21, 2006
Patent Expiration: July 2023
2022 Sales: $2.5B
Indications: Asthma and COPD
Symbicort is a drug that aids in reducing lung inflammation and keeping the airways open. The drug comprises a combination of ICS/budesonide (anti-inflammatory) and LABA/formoterol fumarate (helps relax smooth muscles around airways). Last year Viatris (previously Mylan) and Kindeva’s Breyna earned ANDA the US FDA approval as the first generic version of Symbicort.

Company: Merck
Generic Name: Sitagliptin
First Approval Date: Oct 17, 2006
Patent Expiration: Jan 2023
2022 Sales: $2.8B
Indication: Type 2 Diabetes
Januvia is an anti-diabetic drug indicated with diet and exercise in patients with Type-2 diabetes. It is a once-daily prescription pill that prolongs the action of GIP and GLP-1, enhancing the active incretin levels increasing insulin production, and decreasing hepatic glucose overproduction. Many companies have received the US FDA approval for their Januvia generic version expecting rollout once Merck's patent expires.

Company: Roche
Generic Name: Tocilizumab
First Approval Date: Jan 11, 2010
Patent Expiration: Jan 2023
2022 Sales: $2.9B
Indications: Rheumatoid Arthritis, Giant Cell Arthritis, Juvenile Idiopathic Arthritis, COVID-19
Actemra is an immunosuppressive therapy targeting human interleukin (IL-6) receptors. Though the exact working of Actemra in the body is not known, from early research, it is suggested that Actemra blocks the connecting of IL-6 protein to the cells and prevents overactivity of the immune system. As of now, there are no the US FDA approved biosimilars available for Actemra. However, last August, Fresenius Kabi announced the US FDA's acceptance of its biosimilar BLA, bringing a potential candidate into the market before long. Prospects from Celltrion and Biogen are also in late-stage testing, according to a Cardinal Health report.

Company: Shire (Takeda Pharmaceuticals)
Generic Name: Lisdexamfetamine
First Approval Date: Feb 23, 2007
Patent Expiration: Aug 2023
2022 Sales: $3.12B
Indication: Attention-Deficit/Hyperactivity Disorder (ADHD) and Binge Eating Disorder (BED) in adults
Vyvanse is a CNS stimulant with the active ingredient Lisdexamfetamine (a prodrug of dextroamphetamine). After oral administration, Lisdexamfetamine is absorbed rapidly from the GI tract and circulated into the bloodstream. Due to the hydrolytic activity of RBCs, Lisdexamfetamine gets converted into dextroamphetamine and l-lysine. Dextroamphetamine blocks the reuptake of dopamine and catecholamines norepinephrine into the presynaptic neuron and increases monoamines release into the extraneuronal space hence treating ADHD and BED. The drug is offered in strengths of 10 mg – 70mg as chewable tablets and capsules. Currently, Vyvanse does not have a therapeutically equivalent version in the US. However, the US FDA records state companies like Teva, Amneal, Sandoz, and others are anticipating tentative generic approvals.

Company: Novartis
Generic Name: Sacubitril-Valsartan
First Approval Date: Jul 7, 2015
Patent Expiration: Apr 2023
2022 Sales: $4.64B
Indication: Chronic Heart Failure
Entresto is a prescription medicine developed for treating long-lasting (chronic) heart failure in adults. The drug comprises two active ingredients: valsartan (reduces tightening of blood vessels and building up of sodium and fluid) and sacubitril (helps relax blood vessels and decrease sodium and fluid in the body). The combination of both drugs sets Entresto apart, making it the number one heart failure brand prescribed by cardiologists. Entresto is available as film-coated tablets in 24/26 mg, 49/51 mg, and 97/103 mg. No generic version of Entresto has been approved in the US.

Company: J&J
Generic Name: Ustekinumab
First Approval Date: Sep 25, 2009
Patent Expiration: Sep 2023
2022 Sales: $9.72B
Indications: Plaque Psoriasis, Active Psoriatic Arthritis, Crohn’s disease, and Ulcerative Colitis
Stelara is a biologic targeting an overactive immune system. The only the US FDA approved biologic prevents the elevation of two human interleukins, IL-12 and IL-23 proteins associated with gastrointestinal inflammation. By blocking these proteins, Stelara also aids in reducing inflammation that causes joint pain. Mostly, ROA for Stelara is via subcutaneous injections, although adult patients of Ulcerative Colitis and Crohn’s disease receive the first dose as IV. Currently, there are no the US FDA-approved biosimilars in the US. However, candidates from Amgen and Alvotech are under the US FDA review (decisions are expected around Sep 1 and Nov 6, respectively), and another six biosimilars are in development. However, in Japan, Fuji Pharma is commercializing Alvotech’s Stelara biosimilar.

Company: AbbVie
Generic Name: Adalimumab
First Approval Date: Dec 31, 2002
Patent Expiration: Jan 2023
2022 Sales: $21.37B
Indications: Rheumatoid Arthritis, Crohn’s Disease, Plaque Psoriasis, Hidradenitis Suppurativa
Humira is a recombinant human IgG1 monoclonal antibody injected under the skin by a pre-filled syringe or an auto-inject pen. Humira binds to TNF-alpha molecules and blocks their interaction with the p75 and p55 cell surface TNF receptors. As a result, in Arthritis patients, Humira reduces joint pain, swelling, and stiffness and stops further irreversible joint damage. With the top drug in the industry facing a patent cliff, around 8 Humira biosimilars are expected to launch in the US in 2023, namely Amjevita (Amgen), Abrilada (Pfizer), Cyltezo (BI), Hadlima (Organon/Samsung Bioepis), Yusimry (Coherus Biosvinecs), Idacio (Fresenius Kabi) and Hyrimoz (Sandoz).
Sources:
- Annual reports
- SEC Filings
- Press releases
- Company websites
Note:
- All revenues are reported in $B and $M
We have taken a fiscal year as per the US
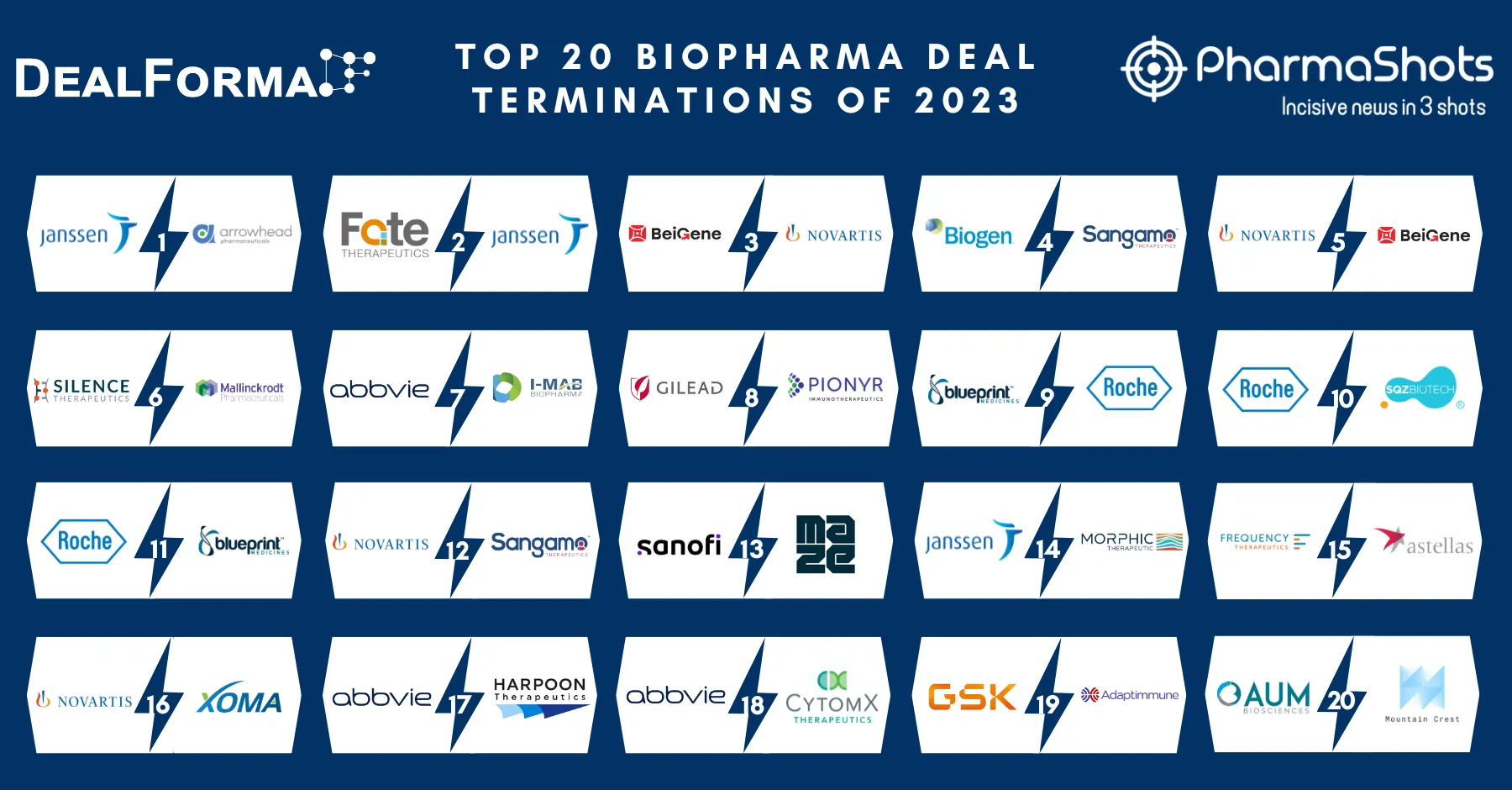
Related Post: Top 20 Biopharma Deal Terminations of 2022 Based on Total Deal Value
Tags

Shivani is a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She covers news related to Product approvals, clinical trial results, and updates. She can be contacted at connect@pharmashots.com.