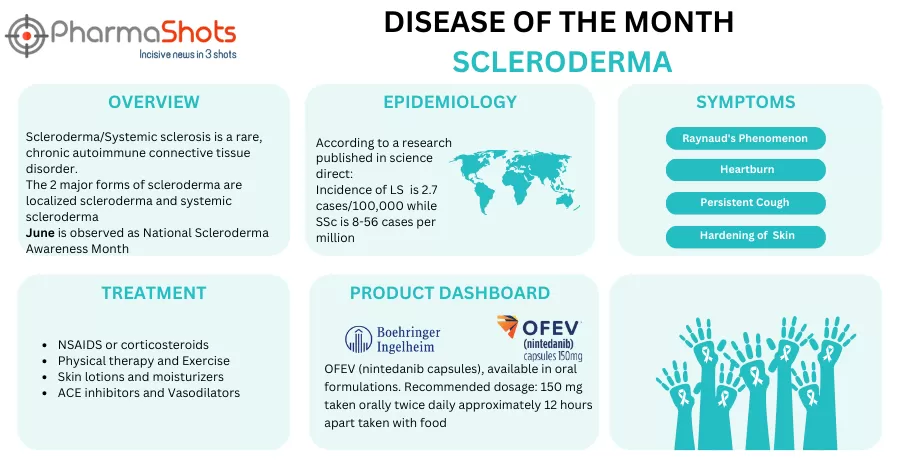
Kyverna Therapeutics Receives the US FDA’s IND Clearance to Initiate the P-I/II Study of KYV-101 for the Treatment of Scleroderma
Shots:
- The US FDA has cleared the third IND application to initiate a P-I/II study of KYV-101, an autologous fully human anti-CD19 CAR T cell therapy for the treatment of diffuse cutaneous systemic sclerosis (scleroderma)
- KYV-101 is currently being studied in an ongoing P-I trial (KYSA-1) in the US and a P-I/II trial (KYSA-3) in Germany for adults with active lupus nephritis. The therapy is designed to modify a patient's T cells to target CD19
- In a P-I/II trial in oncology, KYV-101 demonstrated anti-lymphoma activity along with a significant reduction of cytokines. Kyverna gained exclusive, global licenses from the NIH to use this CD19 construct in autologous as well as allogeneic CAR T-cell therapies
Ref: PRNewswire | Image: Kyverna Therapeutics
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com
Click here to read the full press release

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.