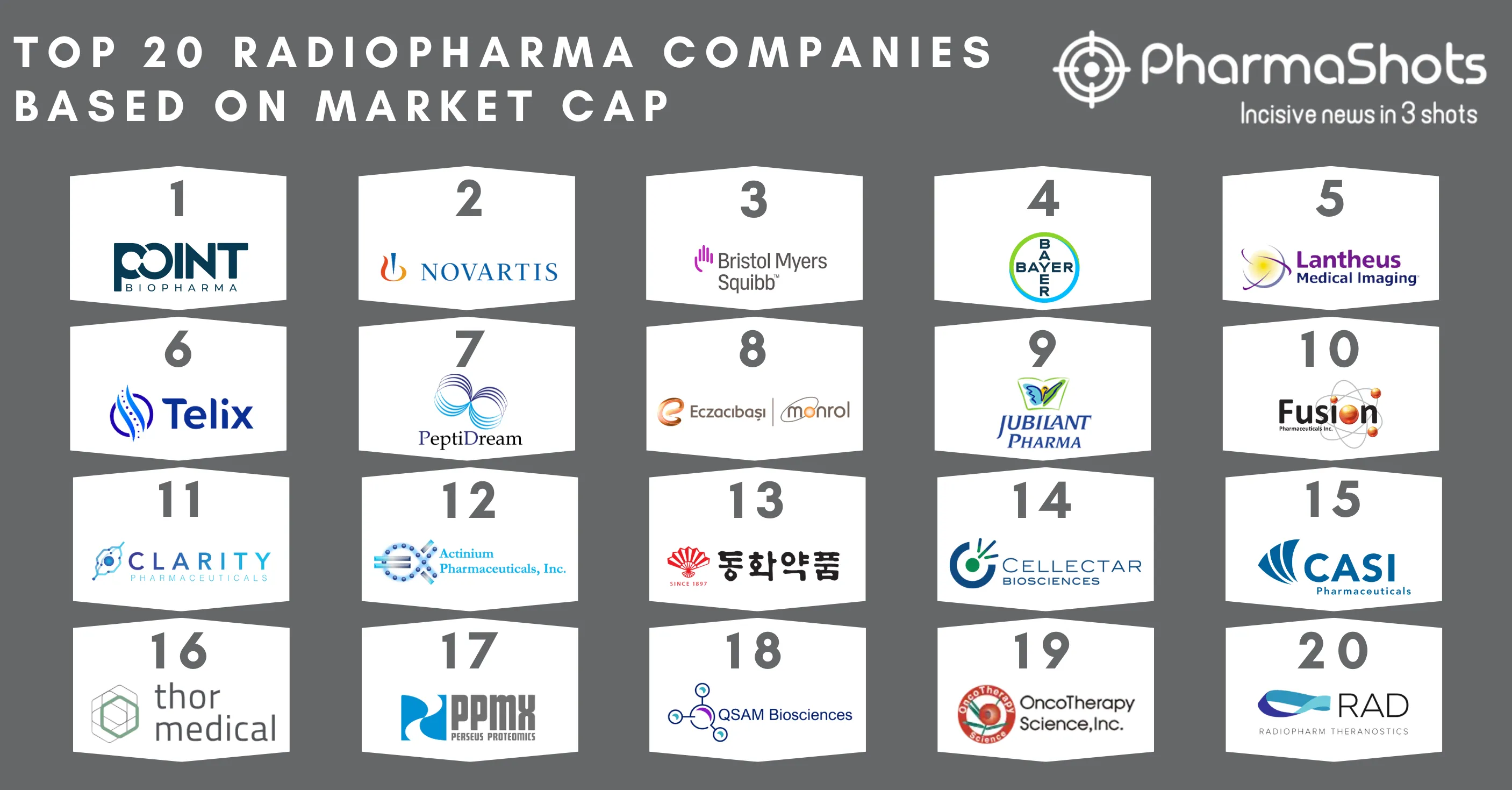
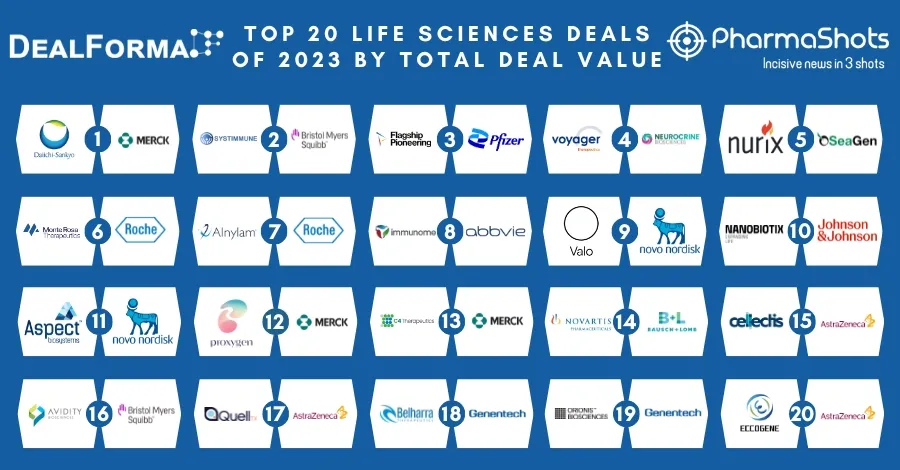
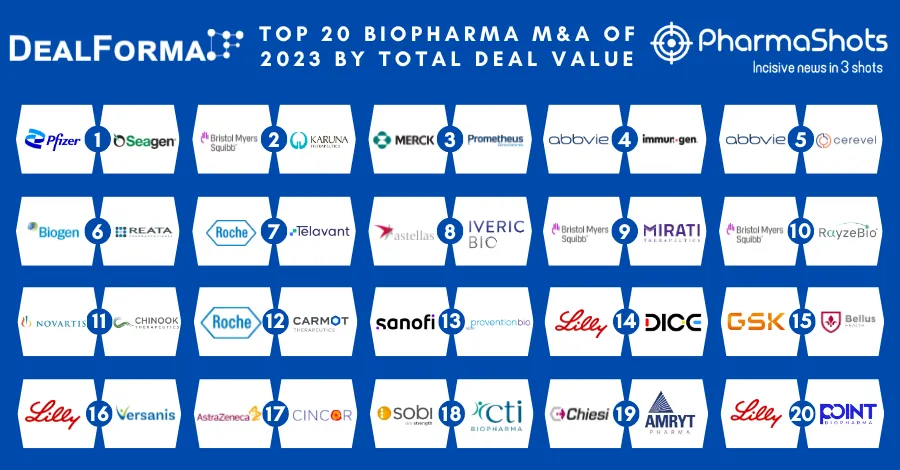
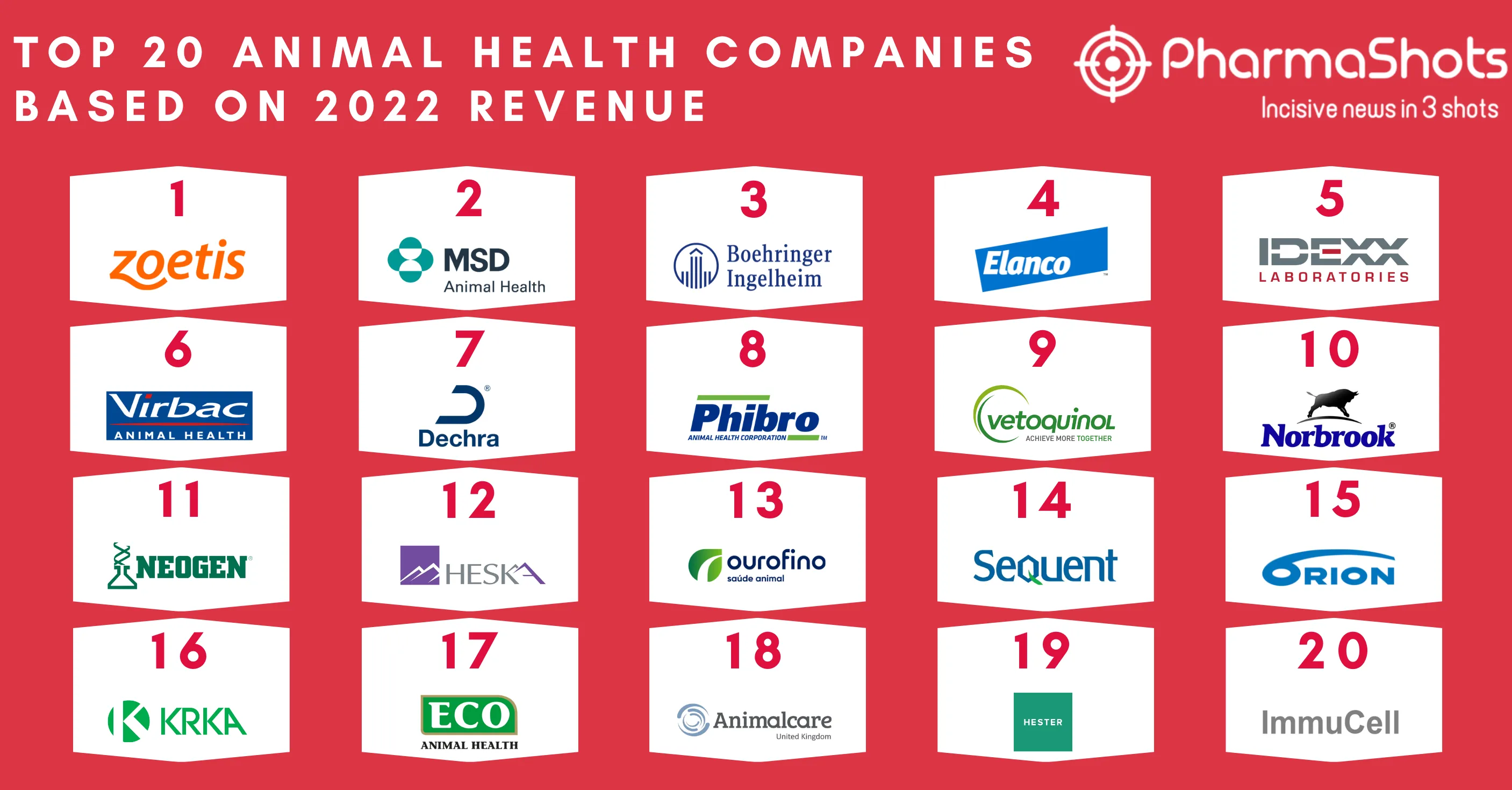
Top Biosimilar Companies with Approved and Pipeline Products in the US and EU
Biosimilars are the key alternatives for expensive Biologics therapies and saving millions of dollars of patients spent in the treatment of multiple diseases. Hence physicians are likely to adopt biosimilars a reference product to biologics possessing similar therapeutic properties in terms of potency- safety- and efficacy to original biologic products. The companies are focusing on the expansion of their pipeline and portfolio in Biosimilars. In 2018- Amgen collaborated with Orion for the commercialization of Amgevita (biosimilar- adalimumab) in Finland in addition to STADA's joint development agreement with Xbrane Biopharma for Xlucane (biosimilar- ranibizumab). Biocon also came up with CHMP recommendations for its biosimilars Fulphila (biosimilar- pegfilgrastim) and Ogivri (biosimilar- trastuzumab) targeted to treat Neutropenia and HER-2 Breast Cancer respectively. Our team at PharmaShots has compiled a list of top biosimilar companies developing and marketing biosimilars in the EU and the US arranged in alphabetical orders.

Amgen
Total Biosimilar Products: 9 Biosimilar Pipeline Products: 4
Founded Year: 1980 Headquarter: United States
Market Cap: $139.36B Stock Exchange: NASDAQ
Amgen is one of the leading biotechnology company developing novel therapies focused on Cardiology- Oncology- Immunology- Neurology- Nephrology- and Inflammatory diseases. In Oct 2018 Amgen signed an agreement with Orion for the commercialization of Amgevita (adalimumab- biosimilar) in Finland. Additionally- in H2'18 Amgen also launched its first inflammation biosimilar Amgevita (biosimilar- adalimumab) in Europe for the treatment of inflammatory diseases including all possible indications for Humira (adalimumab). Amgen's ABP 710 (biosimilar infliximab) reported results of P-III study in comparison with Remicade (infliximab) for patients with moderate-to-severe rheumatoid arthritis with its BLA submitted to the FDA. Amgen's ABP 798 (biosimilar- rituximab) has completed its P-III studies for moderate to severe RA & NHL and the trial evaluating ABP 959 (biosimilar- eculizumab) is recruiting patients for P-III for Paroxysmal nocturnal hemoglobinuria (PNH).

Apotex/Apobiologix
Total Biosimilar Products: 5 Biosimilar Pipeline Products: 5
Founded Year: 1974 Headquarter: Canada
Market Cap: NA Stock Exchange: NA
Apotex is a generics drugs company- headquartered in Canada. Apobiologix is a member of the Apotex Group of Companies- focused on the development of biosimilars. Apobiologix has launched its pegfilgrastim biosimilar- Lapelga- in Canada in Q1'19. Apobiologix has been developing 5 products for the US market- including Epoetin alfa- Darbepoetin alfa- Bevacizumab- Rituximab- and Trastuzumab.

Biocon
Total Biosimilar Products: 5 Biosimilar Pipeline Products: 4
Founded Year: 1978 Headquarter: India
Market Cap: ~$4.29B Stock Exchange: NSE
Biocon is a global pharmaceutical company involved in discovering- developing and producing therapies in chronic therapies such as Diabetes- Oncology- and Immunology. The vast portfolio of Biocon can be further divided into APIs and Generic formulations- Biologics & Biosimilars and Branded Formulations. In Sept 2018- Biocon and Mylan's Fulphila (biosimilar- pegfilgrastim) received CHMP recommendation for neutropenia. Biocon's Insulin Aspart and Krabeva (biosimilar- bevacizumab) are targeted for diabetes and multiple indications in cancer respectively while they are currently being evaluated in P-III study.

Biogen
Total Biosimilar Products: 3 Biosimilar Pipeline Products: 2
Founded Year: 1978 Headquarter: United States
Market Cap: ~$54.74B Stock Exchange: NASDAQ
Biogen is a US-based biotech company focused on the development of therapies in neuroscience particularly in multiple sclerosis and neuroimmunology- neuromuscular disorders- movement disorders- ophthalmology- immunology- neurocognitive disorders- acute neurology- and pain. In 2018- the company also reported pooled results of P-III studies for Benepali (biosimilar- etanercept)- Flixabi (biosimilar- infliximab)- and Imraldi (biosimilar- adalimumab) in patients with moderate to severe rheumatoid arthritis. In 2019- Biogen signed an agreement with Samsung Bioepis to acquire exclusive commercialization rights for SB11 (biosimilar- ranibizumab) in the US- EU- Japan- Australia and Canada including Benepali- Flixabitm- and Imraldi in China.

Boehringer Ingelheim
Total Biosimilar Products: 15 Biosimilar Pipeline Products: 12
Founded Year: 1885 Headquarter: Germany
Market Cap: N/A Stock Exchange: N/A
Boehringer Ingelheim is a Germany based global pharmaceutical company focused on developing therapies for multiple therapy areas including Cardiovascular- Oncology- CNS- Respiratory- Immunology- Metabolic diseases- and Retinal Health. In 2018- BI reported P-III results of Cyltezo (biosimilar- adalimumab) in patients with moderate-to-severe plaque psoriasis. Additionally- BI has BI 695502 (biosimilar- bevacizumab) in P-III targeted for the treatment of patients with advanced non-squamous NSCLC.

Celltrion
Total Biosimilar Products: 6 Biosimilar Pipeline Products: 1
Founded Year: 2002 Headquarter: South Korea
Market Cap: $20.189B Stock Exchange: KRX
Celltrion is a Korea based healthcare company offering development and sales of the biosimilar in fields of Autoimmune Disorders and Oncology. In Dec 2018 Celltrion's CT-P10 (biosimilar- rituximab) showed well-tolerated and safe results in P-III in patients with advanced follicular lymphoma (AFL). In 2018- Celltrion and Teva's Herzuma (CT-P6- biosimilar- trastuzumab) and Truxima (rituximab-abbs) also received the FDA's approval for HER2-overexpressing breast cancer and three NHL indications respectively. Additionally- Celltrion's CT-P16 (biosimilar- bevacizumab) is also evaluated in P-III trial for treating Colorectal Cancer.

Coherus Biosciences
Total Biosimilar Products: 4 Biosimilar Pipeline Products: 3
Founded Year: 2010 Headquarter: United States
Market Cap: ~$1.35B Stock Exchange: NASDAQ
Coherus is a leading biosimilar company focused on developing candidates in multiple therapeutic areas including oncology- immunology- and ophthalmology. In 2018- Coherus Udenyca (biosimilar- pegfilgrastim) received the EU's MAA and the US FDA's approval for febrile neutropenia and patients receiving myelosuppressive chemotherapy respectively. Coherus' two biosimilars CHS-1420 (biosimilar- adalimumab) and CHS-0214 (biosimilar- etanercept) have also completed their P-III studies for psoriasis and RA respectively.

Fresenius Kabi
Total Biosimilar Products: 6 Biosimilar Pipeline Products: 4
Founded Year: 1999 Headquarter: Germany
Market Cap: N/A Stock Exchange: N/A
Fresenius Kabi is a global healthcare company with a portfolio of IV generic drugs- infusion therapies- and clinical nutrition products including devices for administration of these drugs and biosimilars focusing on Oncology and autoimmune disorders. In 2018- the company signed a settlement and license agreement with AbbVie for its all pending patent litigations regarding the commercialization of Fresenius MSB11022 (biosimilar- adalimumab) in the US. In late 2018- Fresenius also released the results of its MSB11455 (biosimilar- pegfilgrastim) meeting all 1EPs.

Fujifilm Kyowa Kirin Biologics
Total Biosimilar Products: 3 Biosimilar Pipeline Products: 2
Founded Year: 1934 Headquarter: Japan
Market Cap: ~$24.4B Stock Exchange: TYO
Fujifilm Kyowa Kirin Biologics is a Japan-based biopharmaceutical company focused on developing biosimilars targeting Immunology & Oncology. The company leverages Kyowa Kirin's R&D and production expertise- manufacturing- quality management- and analytical skills along with Fujifilm wide-ranging business to create an innovative production process leading to a reduction in production cost.- In 2018- Kyowa Kirin and Mylan collaborated to commercialize Hulio (FKB327) (biosimilar- adalimumab) and has also received EMA's marketing authorization for Rheumatoid Arthritis- Psoriasis- Crohn's Disease.

Gedeon Richter
Total Biosimilar Products: 2 Biosimilar Pipeline Products: 2
Founded Year: 1901 Headquarter: Hungary
Market Cap: 3.174B Stock Exchange: Budapest Stock Exchange
Gedeon Richter is a Budapest based company- focused on developing therapies for multiple therapeutic areas including Women's Healthcare- Central Nervous System- and Cardiovascular areas. In early 2018- EMA initiated the evaluation of Richter's resubmitted marketing authorization application for proposed biosimilar pegfilgrastim. In 2018- Richter acquired IPR for Bemfola for use in the US.

Intas/Accord Healthcare
Total Biosimilar Products: 6 Biosimilar Pipeline Products: 6
Founded Year: 1968 Headquarter: Ahmedabad- India
Market Cap: NA Stock Exchange: NA
Intas is a pharmaceutical company focused on developing therapies for CNS- CVS- Oncology- Diabetes- Gastroenterology- Pain Management- Urology- Nephrology- Gynecology- Infertility- and Respiratory Care. Intas biosimilars are developed at the company's European Union-Good Manufacturing Practices (EU-GMP) certified biotechnology plant situated near Ahmedabad.

Mabion
Total Biosimilar Products: 3 Biosimilar Pipeline Products: 3
Founded Year: 2007 Headquarter: Poland
Market Cap: ~$1.34B Stock Exchange: WSE
Mabion is a Poland based leading biopharmaceutical company developing and commercializing biosimilar and drugs based on its monoclonal antibody technology. In 2018- Mabion filed marketing authorization for its Mabion CD20 (biosimilar- rituximab) candidate- targeted to treat RA patients and patients with non-Hodgkin's lymphoma.

Merck & Co.
Total Biosimilar Products: 3 Biosimilar Pipeline Products: 0
Founded Year: 1894 Headquarter: United States
Market Cap: ~$222.37B Stock Exchange: NYSE
Merck is a global healthcare leader focused on developing- discovering and providing prescription medicines- vaccines- biologic therapies- and animal health products. In 2018- Merck Renflexis (biosimilar- infliximab) determined to be of the lowest-priced infliximab option available and was also awarded a national contract by the VA. With the approval- in 2018 Merck also terminated its deal with Samsung Bioepis for Lusduna (biosimilar- insulin glargine) and paid $155M as a termination fee.
Related Post: Top 20 Biopharma Acquisitions of 2018 Based on the Total Deal Value
Tags

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com