After Effects of Russia – Ukraine Conflict Over Pharma and Life Sciences Industry
Everywhere on TV, social media, digital print or print media we are hearing/ watching how Russia has invaded Ukraine. Russia and Ukraine are at war which is impacting not only both companies but also millions of lives globally.
The situation has affected many areas of day-to-day life including a big burden on the healthcare system and specifically is having a significant impact on clinical trials. To understand the impact on clinical trials due to war, needs a thorough understanding and analysis of clinical trials. Clinical trials include series of events where patients are screened, recruited, dossed, followed-up, tracked & monitored to assess the safety and efficacy of drug in patients and then based on the data collected, regulatory agencies issue approvals.
Lately, Ukraine has been one of the prime destinations for conducting clinical trials because Ukraine has a well established centralized healthcare system which helps various pharmaceutical companies in easy and faster recruitment. In addition, Ukraine has a large treatment naïve patient pool with GCP complaint staff and infrastructure1.
With war ongoing, there are multiple situations which are affecting the whole clinical trials system:(a) unavailability of patients new and ongoing both, (b) blocked supply chain which will and has overall affected pharmaceutical industry. In these circumstances, there can be two different ways to tackle the situation.
- Firstly, recruit new patients in other countries (already existing or new), but this will increase burden on the companies including monetary investments and this might lead to delay in results and then there is a big question for later what will happen to the patients who have been recruited and dosed
- Secondly, if companies wait for the dispute to settle but no one knows for how long? If the situation goes on for a little while, then what will happen to the data for patients who had a gap in their dosing schedule. In addition, there could be a possibility of psychological effects of war on patients which might lead to unrelated adverse effects which could later effect on the overall trial results. So, there are multiple questions which have no right answers but only ripple effect
We all live in a data driven world. So, let’s see some numbers which may help understand the severity of the issue
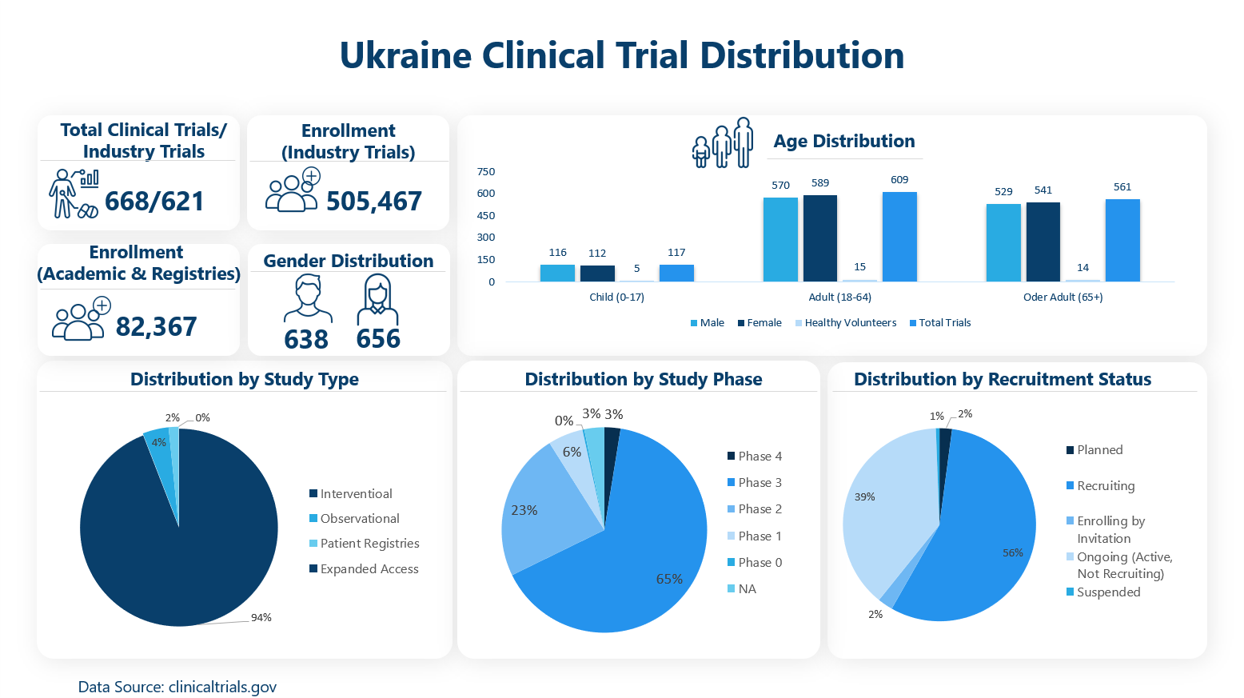
So far there are 668 trials in total, out of which 621 are industry-sponsored trials, 49 are non-industry (incl. expanded access, patient registries etc.) trials and 2 are sponsored by both industry and non-industry institutions.

From this analysis, we have picked top 10 companies based on number of ongoing trials in Ukraine.
- J&J has the maximum number of ongoing studies in Ukraine (n=60) of which 36 trials are being assessed in phase 3 (n=36) maximum trials being conducted for oncology indications (n=17)
- Roche (n=58) & MSD (n=57) follows J&J in terms of number of studies being conducted in Ukraine with phase 3 distribution of (n=48) & (n=46) respectively
- Both companies are conducting maximum trials for oncology indications with (n=32) & (n=40) respectively
- There are 206 unique pharma/ biotech companies conducting ~621 studies in Ukraine

- Oncology is the most assessed therapy area with total 232 ongoing studies, out of which 174 trials are phase 3 studies
- Out of 232 oncology studies, maximum ongoing trials are being conducted for NSCLC (n=50) recruiting ~29,007 patients across Ukraine followed by trials in auto-immune category with 81 ongoing studies recruiting ~48,166 patients
References
- Reasons to choose Ukraine as the location for your next clinical trial. Cromos Pharma PR 16-Mar-2021 (Source)
- Data collected from Clinicaltrials.gov



