
Top Performing Drug of 2021 – Stelara (August Edition)
Active Ingredients: Ustekinumab
Strength: 45 mg/0.5 mL or 90 mg/mL (SC), 130 mg/26 mL (IV)
Dosage Form: Injection
Mechanism of Action: Interleukin 12 & 23 inhibitor
First Approval: US (25 Sep, 2009), EU (16 Jan, 2009)
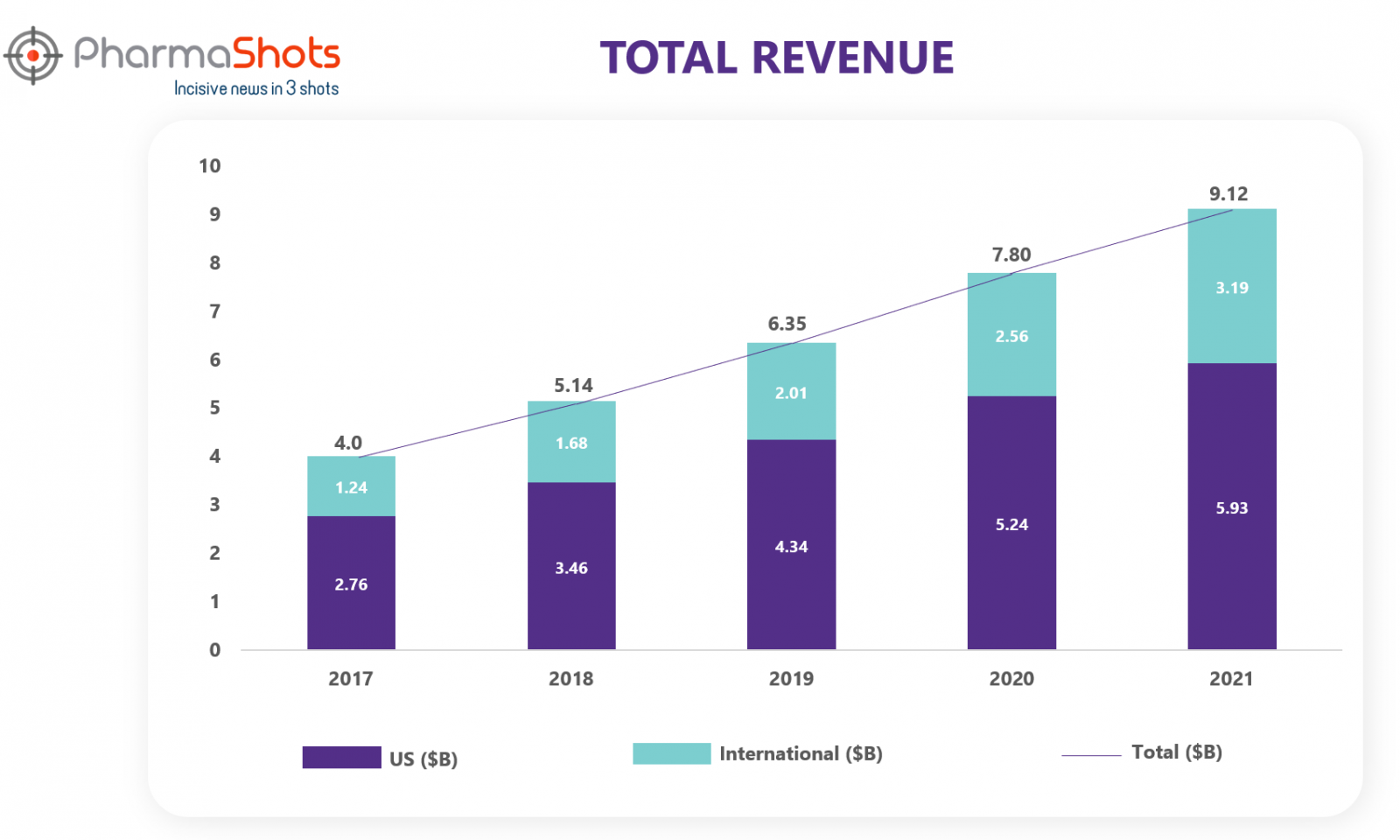
Revenue1
Stelara is the leading blockbuster drug of Johnson & Johnson. Stelara contributed 9.7% of the Company's total revenues for 2021. Stelara up took the immunology segment revenue for J&J. Stelara is sold globally including in the US, the UK, France, Germany, and Japan. Worldwide percentage sales increased by 18.5% (2021), 21.10% (2020), 23.40% (2019), 28.50% (2018) and 24.1% (2017). Let’s explore in what manner the revenue of Stelara changed over the last five years.
Approved Indication for Stelara2
STELARA is a human interleukin-12 and -23 antagonist indicated for the treatment of:
Adult patients with:
- Plaque psoriasis: Moderate to severe plaque psoriasis (Ps) who are candidates for phototherapy or systemic therapy
- Active psoriatic arthritis
- Crohn’s disease: Moderately to severely active Crohn’s disease (CD)
- Ulcerative colitis: Moderately to severely active ulcerative colitis
Paediatric patients 6 years and older with:
- Plaque psoriasis: Moderate to severe plaque psoriasis, who are candidates for phototherapy or systemic therapy
- Active psoriatic arthritis (PsA)
Clinical Trials3
Stelara has a total of 197 trials, including 136 industry trials of which 103 were interventional, 32 observational & 1 expanded access trial. From the analysis of clinical trials, we have made a representation for the trials of Stelara (Trials are taken as of 18 Aug 2022)
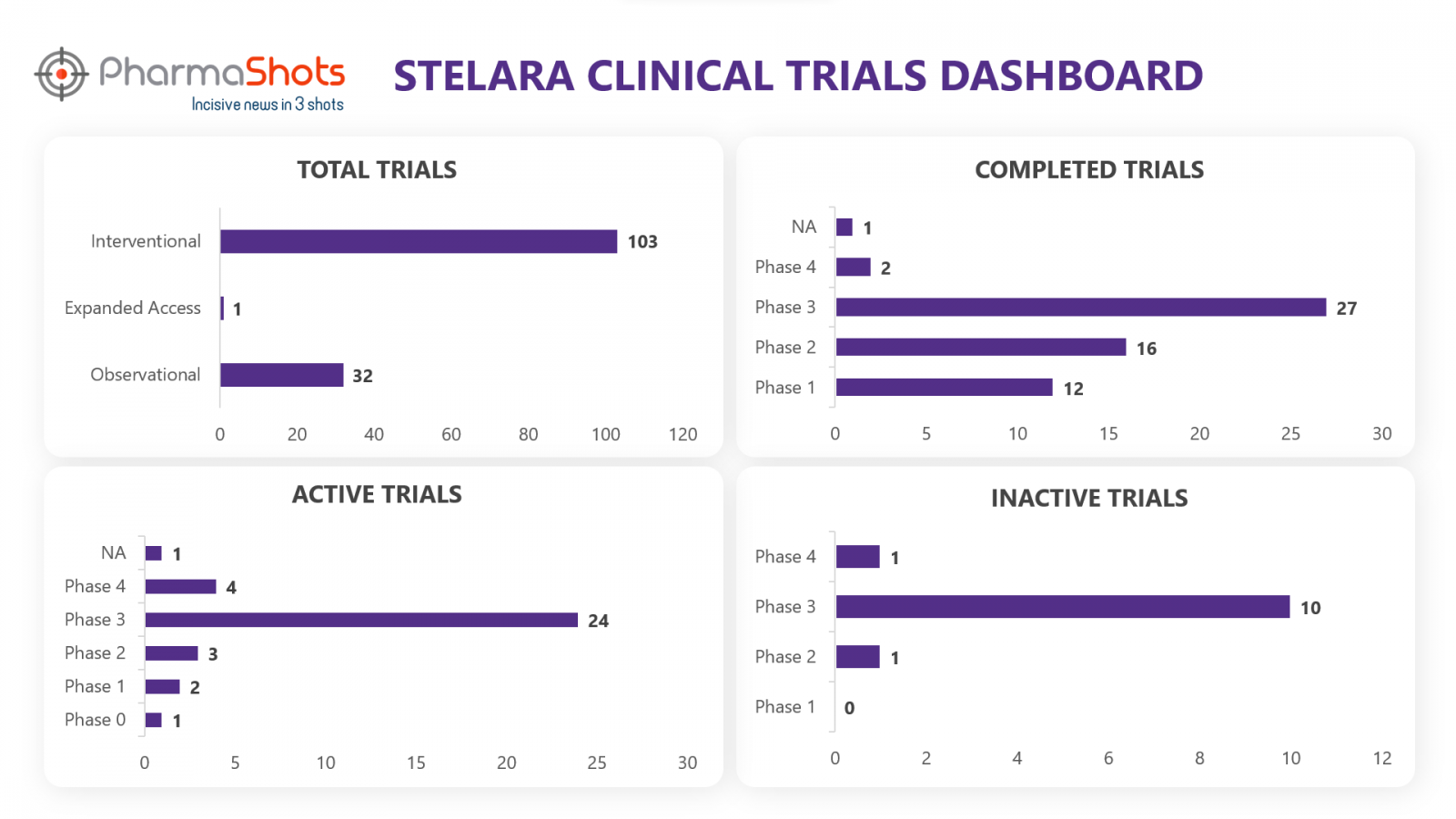
*Active trials include Recruiting; Active, Not Recruiting; Enrolling by Invitation, and suspended
*Inactive trials include Terminated; Withdrawn; Unknown Status
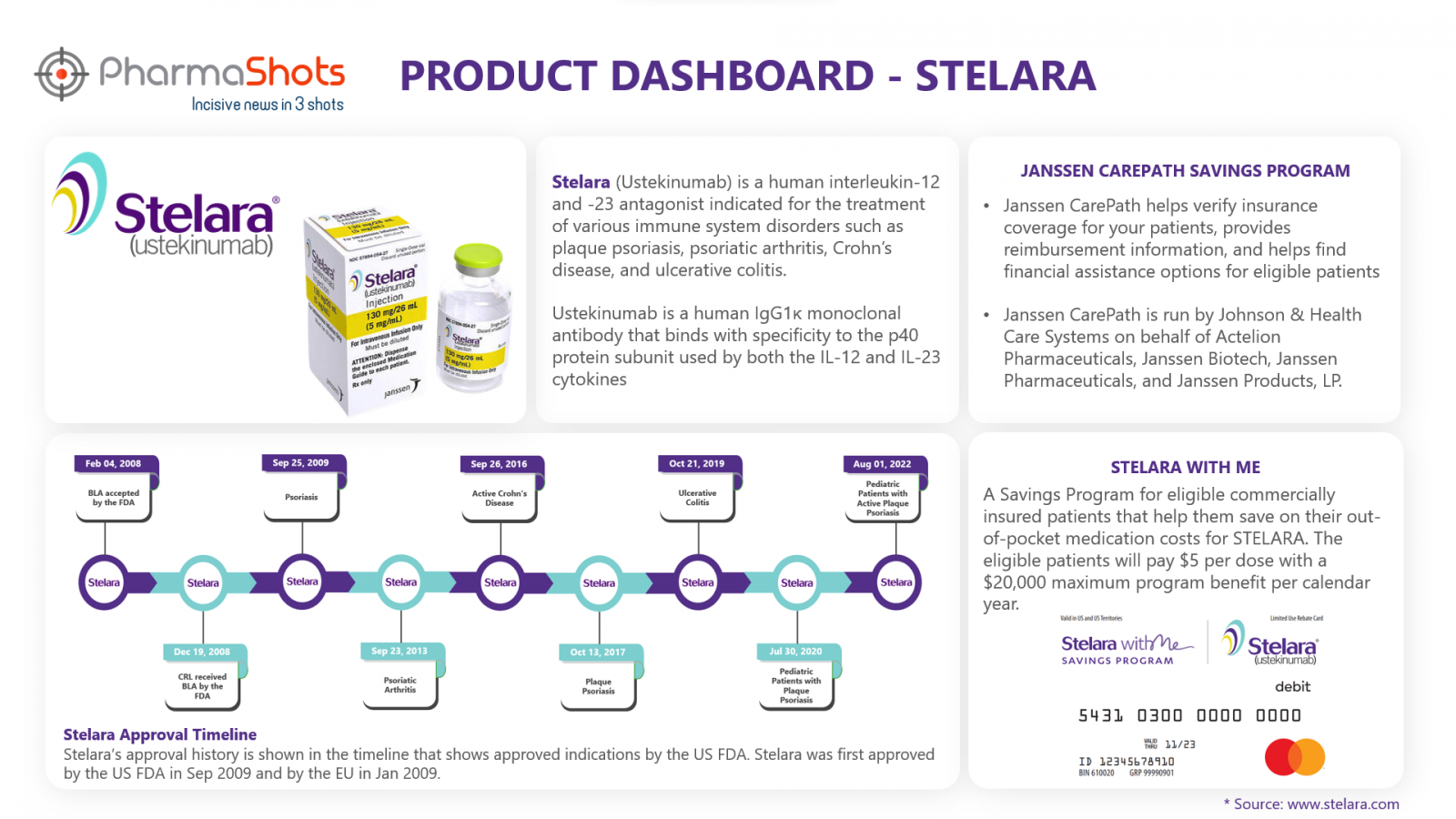
Janssen CarePath Savings Program4
Janssen CarePath helps patients verify insurance coverage and provides reimbursement information that helps them to find financial assistance options. Janssen CarePath program provides several benefits for the patients:
- Conduct health plan coverage review
- Help identify financial assistance options for eligible patients
- Provide care coordination with a treatment provider or pharmacy
- Provide patient support resources
Janssen CarePath Provider Portal
Janssen CarePath Provider Portal is developed in collaboration with IBM Watson Health. The Provider Portal provides 24-hour online access to not only enroll eligible but also commercially insured patients in the Janssen CarePath Savings Program. It helps them to view and manage their Savings Program benefits
Stelara with me5
A Savings Program for eligible commercially insured patients that help them save on their out-of-pocket medication costs for STELARA. This program also provides some optional support listed below:
- Nurse Navigator: After enrolment, a dedicated Nurse Navigator (a registered nurse) is assigned who will contact the patients. Nurse Navigator is ready to offer support and answer the questions about STELARA, from how to self-inject to how to make the medication more affordable
- Treatment Support: The Nurse Navigator can help schedule one-time IV infusion and provides self-injection training, and offer support to help stay on track throughout the treatment journey
- Prescription & Cost Support: Patents can verify the insurance coverage and understand how to fill their prescriptions, and look for options that could make the treatment more affordable
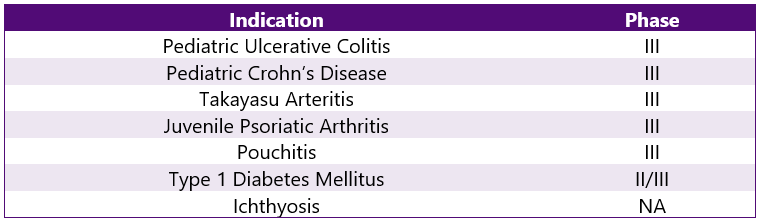
Stelara Pipeline Analysis6
Stelara is being studied under various trials ongoing for other indications. This can be categorized as follows:
Other information:
Patents7
Patents are a key determinant of market exclusivity for most branded pharmaceuticals. Many companies face patent challenges from third parties, including challenges seeking to manufacture and market generic and biosimilar versions of the Company's key pharmaceutical products. The US patent for Stelara expires in 2023 and the European composition of matter patent expires in 2024.
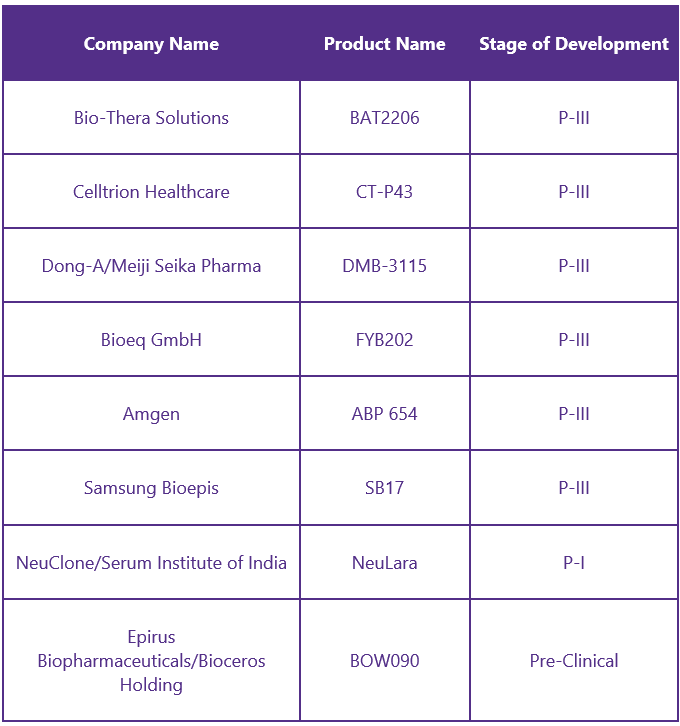
Biosimilars8
The development of Stelara biosimilars will allow more patients to access this anti-inflammatory therapy, which will lead to significant improvements in their quality of life. The expiration of Stelara’s patent in 2023 has led to an increasing number of biosimilars. Some of its biosimilars include:
References:
2. Stelara Prescribing Information
4. Janssen CarePath Savings Program
6. Johnson & Johnson – Pipeline
Related Post: Top Performing Drug of 2021 - Eliquis (July Edition)
Tags

Senior Editor at PharmaShots. She is curious and very passionate about recent updates and developments in the life sciences industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots.













