Insights+: EMA Marketing Authorization of New Drugs in August 2022
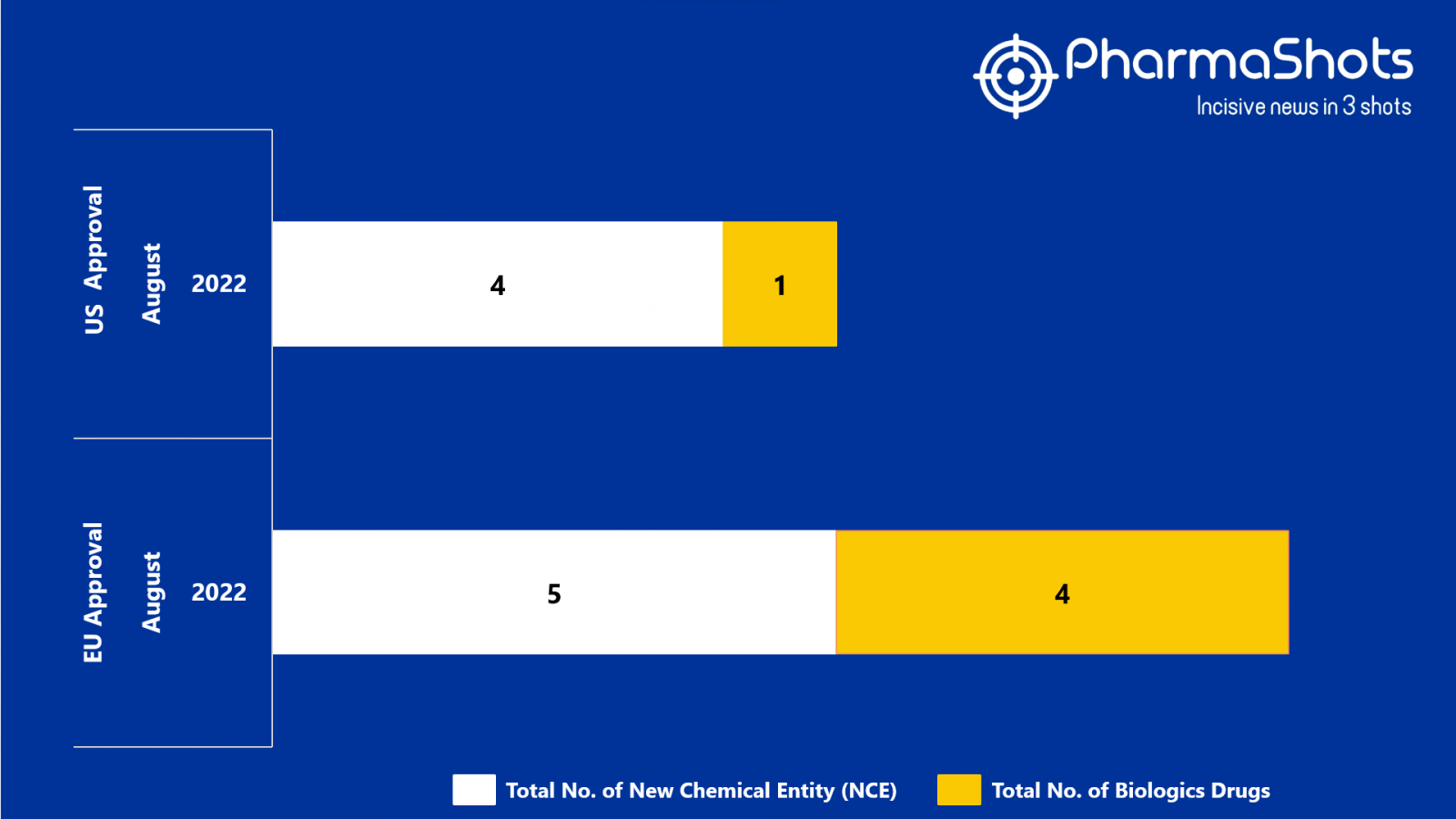
- The EMA approved 5 New Chemical Entity (NCE) and 4 Biologic Drugs in August 2022, leading to treatments for patients and advances in the healthcare industry
- In August 2022, the major highlights drugs were Lynparza’s approval for breast cancer, Imbruvica (ibrutinib) for chronic lymphocytic leukaemia, Sunlenca (lenacapavir) for HIV Infection
- PharmaShots has compiled a list of a total of 9 new drugs approved by the EMA in August 2022
Lynparza
Active ingredient: olaparib Approved: August 4, 2022
Company: AstraZeneca Disease: Breast Cancer
- The approval was based on the P-III (OlympiA) trial evaluating the efficacy & safety of Lynparza vs PBO in patients with gBRCAm high-risk HER2- early breast cancer prior treated with neoadjuvant or adjuvant CT. The trial was sponsored by NRG Oncology in the US & by AstraZeneca outside the US
- The results showed an improvement in iDFS with a 42% reduction in risk of invasive breast cancer recurrences, new cancers, or death, and an improvement in OS with a 32% reduction in risk of death. The safety & tolerability profile of Lynparza was consistent with that observed in prior trials
- Lynparza was approved in the US for gBRCAm, HER2- high-risk early breast & was also approved in the US, EU, Japan & multiple other countries for gBRCAm, HER2- metastatic breast cancer
Imbruvica
Active ingredient: ibrutinib Approved: August 4, 2022
Company: Janssen Disease: Chronic Lymphocytic Leukaemia
- The EC has granted marketing authorization for the expanded use of Imbruvica + venetoclax (I+V) in adults with prior untreated CLL
- The approval was based on the P-III (GLOW) study that evaluated I+V vs Clb+O in elderly patients aged ≥65yrs. with CLL/SLL which showed that I+V was superior to Clb+O, and improvement in PFS which was consistent across predefined subgroups
- The approval was also based on the FD cohort of the P-II (CAPTIVATE) study which showed deep & durable responses in patients with I+V, incl. those with high-risk features. The results were published in NEJM Evidence and Blood & the safety profile was consistent with safety profiles of I+V, 1.9% discontinued ibrutinib due to AF, and OS data is not mature with a median follow-up of 34mos.
3. Argenx’s Vyvgart Receives the EC’s Approval for the Treatment of Generalized Myasthenia Gravis
Vyvgart
Active ingredient: efgartigimod alfa Approved: August 12, 2022
Company: Argenx Disease: Generalized Myasthenia Gravis
- The approval was based on the results from the P-III (ADAPT) trial evaluating the safety & efficacy of Vyvgart (efgartigimod alfa-fcab) vs. PBO in a ratio (1:1) for 26wks. in patients (n=167) with gMG across North America, Europe & Japan
- The results demonstrated the response of anti-AChR Ab positive gMG patients treated with Vyvgart on the MG-ADL scale (68% vs. 30%) & on the QMG scale (63% vs 14%) vs PBO
- Vyvgart is a human IgGI Ab fragment that binds to the neonatal Fc receptor (FcRN) to reduce the circulating IgG autoantibodies. Vyvgart has previously been approved in the US & Japan for the treatment of gMG whereas argenx & Zai Lab have jointly planned to launch Vyvgart in Canada & China
Pepaxti
Active ingredient: melphalan flufenamide Approved: August 12, 2022
Company: Oncopeptides Disease: Multiple Myeloma
- The EC has granted marketing authorization for Pepaxti in combination with dexamethasone for adult patients with MM who have received three prior lines of therapies
- The marketing authorization was based on the P-II (HORIZON) study to evaluate melflufen + dexamethasone in 157 patients with r/r MM & was also based on the P-III (OCEAN) study. The product is expected to be launched in Q4’22
- The results from the P-II (HORIZON) study showed ORR (28.8%), DoR (7.6mos.), TTR (2.3mos.). The company plans to submit a type II variation in Q4’22 for earlier lines of treatment & the EC’s marketing authorization is valid in all EU member states, EEA countries Iceland, Lichtenstein & Norway
5. Gilead’s Sunlenca (lenacapavir) Receives EC's Approval for the Treatment of HIV Infection
Sunlenca
Active ingredient: lenacapavir Approved: August 22, 2022
Company: Gilead Disease: HIV Infection
- The EC has granted marketing authorization for Sunlenca (capsid inhibitor) in combination with other antiretrovirals for adults with multi-drug resistant HIV inf.
- The MAA was based on the P-II/III (CAPELLA) study to evaluate the antiviral activity of lenacapavir (SC) + optimized background regimen vs PBO in a ratio (2:1) in 36 heavily treated-experienced patients with multi-drug resistant HIV-1 inf. across North America, the EU & Asia. The PDUFA action date is expected on Dec 27, 2022
- The results showed that 83% of patients achieved an undetectable viral load (<50 copies/mL) @52wk. along with a mean increase in CD4 count of 83cells/µL. The MAA will be valid in 27 member states of the EU, Norway, Iceland & Liechtenstein
Tecvayli
Active ingredient: teclistamab Approved: August 22, 2022
Company: Janssen Disease: Multiple Myeloma
- The EC has granted CMA of Tecvayli as monothx. for r/r MM who received 3 prior therapies incl. an immunomodulatory agent, a proteasome inhibitor & an anti-CD38 Ab
- The CMA was based on the P-I/II (MajesTEC-1) study evaluating teclistamab in 165 adult patients which showed that 104 patients achieved an ORR (63%) after a median of 5 prior lines of therapy; VGPR (58.8%); CR (39.4%); median time to 1st confirmed response was 1.2mos. and m-DoR (18.4mos.) & also showed deep & durable responses, m-PFS (11.3mos.) & m-OS (18.3mos.)
- The therapy is being studied in multiple monothx. & combination studies. Under the license agreement with Janssen, OmniAb will receive $10M in milestones at 1st commercial sale of teclistamab in the UK, Italy, Germany, France, or Spain
7. Teva’s Ranivisio (biosimilar, ranibizumab) Receives EC’s Approval for Retinal Diseases
Ranivisio
Active ingredient: ranibizumab Approved: August 29, 2022
Company: Teva Disease: Retinal Diseases
- The EC has granted the marketing authorization for Ranivisio, a biosimilar to Lucentis for adults across 5 indications incl. AMD, visual impairment due to macular oedema secondary to RVO, visual impairment resulting from DME, PDR, and CNV
- The P-III (COLUMBUS-AMD) study showed that ranibizumab was shown to be highly similar to its reference ranibizumab in terms of clinical efficacy, ocular and systemic safety for AMD and other ophthalmology indications
- Teva & Bioeq AG collaborated for the exclusive commercialization of ranibizumab and the product is expected to be available in the EU over the coming year while the treatment is already available in the UK under the tradename Ongavia
Roctavian
Active ingredient: valoctocogene roxaparvovec Approved: August 29, 2022
Company: BioMarin Disease: Severe Hemophilia A
- The EC has granted CMA to Roctavian for sev. hemophilia A in adult patients without a history of factor VIII inhibitors & without detectable Ab to AAV5
- The EC’s decision was based on the clinical development program incl. 2yr. results from the P-III (GENEr8-1) study showed a stable & durable bleed control with a reduction in mean ABR & mean annualized factor VIII infusion rate
- In the ongoing P-I/II dose escalation study, the results included 5 & 4yr. of follow-up from the 6e13 & 4e13vg/kg dose groups while 6e13vg/kg dose was well tolerated with no delayed-onset TRAEs. Roctavian continues to be made available to eligible patients with sev. haemophilia A & the company is expected to resubmit the BLA at the end of Sept. 2022
9. Novartis’ Scemblix (asciminib) Receives EC’s Approval for the Treatment of Chronic Myeloid Leukemia
Scemblix
Active ingredient: asciminib Approved: August 29, 2022
Company: Novartis Disease: Chronic Myeloid Leukemia
- The EC has approved Scemblix for the treatment of Philadelphia chromosome-positive chronic myeloid leukemia in the chronic phase (Ph+ CML-CP) in adult patients prior treated with two or more TKIs
- The approval was based on the P-III (ASCEMBL) trial evaluating Scemblix vs Bosulif which showed an MMR rate (25.5% vs 13.2%), the discontinuation rate due to adverse reactions (5.8% vs 21.1%) at the 24wk. In the 96wk. longer-term follow-up, MMR rate (37.6% vs 15.8%) & the discontinuation rate was 7.7% vs 26.3%
- The approval will be valid for all 27 EU member states, Iceland, Norway & Liechtenstein. The therapy was approved in multiple countries outside the US, incl. Japan, Switzerland & the UK for Ph+ CML-CP
Note: Roctavian and Tecvayli Received EC’s Conditional Marketing Authorization
Related Post: Insights+: The US FDA New Drug Approvals in August 2022

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.