
Phase III RUBY clinical trial demonstrates potential of Jemperli (dostarlimab) plus chemotherapy to redefine the treatment of primary advanced or recurrent endometrial cancer versus chemother
- 72% and 36% reduction in the risk of disease progression or death observed in the dMMR/MSI-H population and overall patient population, respectively
- Clinically meaningful overall survival trend observed at interim analysis
- Results presented in same-day presentations at ESMO Virtual Plenary and SGO Annual Meeting and simultaneously published in The New England Journal of Medicine
- Regulatory submissions planned for the first half of 2023
GSK plc (LSE/NYSE: GSK) today announced interim results from Part 1 of the RUBY/ENGOT-EN6/GOG3031/NSGO phase III trial investigating Jemperli (dostarlimab) plus standard-of-care chemotherapy (carboplatin-paclitaxel) followed by dostarlimab compared to chemotherapy plus placebo followed by placebo in adult patients with primary advanced or recurrent endometrial cancer.
Hesham Abdullah, Senior Vice President, Global Head of Oncology Development, GSK said: “These positive results from the RUBY trial bring us one step closer to addressing the significant unmet needs of endometrial cancer patients and add to the growing body of evidence on dostarlimab, strengthening our belief in its potential to transform cancer treatment as a backbone immuno-oncology therapy.”
A statistically significant and clinically meaningful improvement in progression free survival (PFS) was observed for dostarlimab plus carboplatin-paclitaxel in the mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H) population (n=118) and in the overall population (n=494) versus placebo plus chemotherapy. The separation of the lines in the Kaplan-Meier curve below illustrates the significant reduction in risk of disease progression or death in patients with dMMR/MSI-H primary advanced or recurrent endometrial cancer in the dostarlimab plus chemotherapy treatment arm compared to the placebo plus chemotherapy treatment arm.
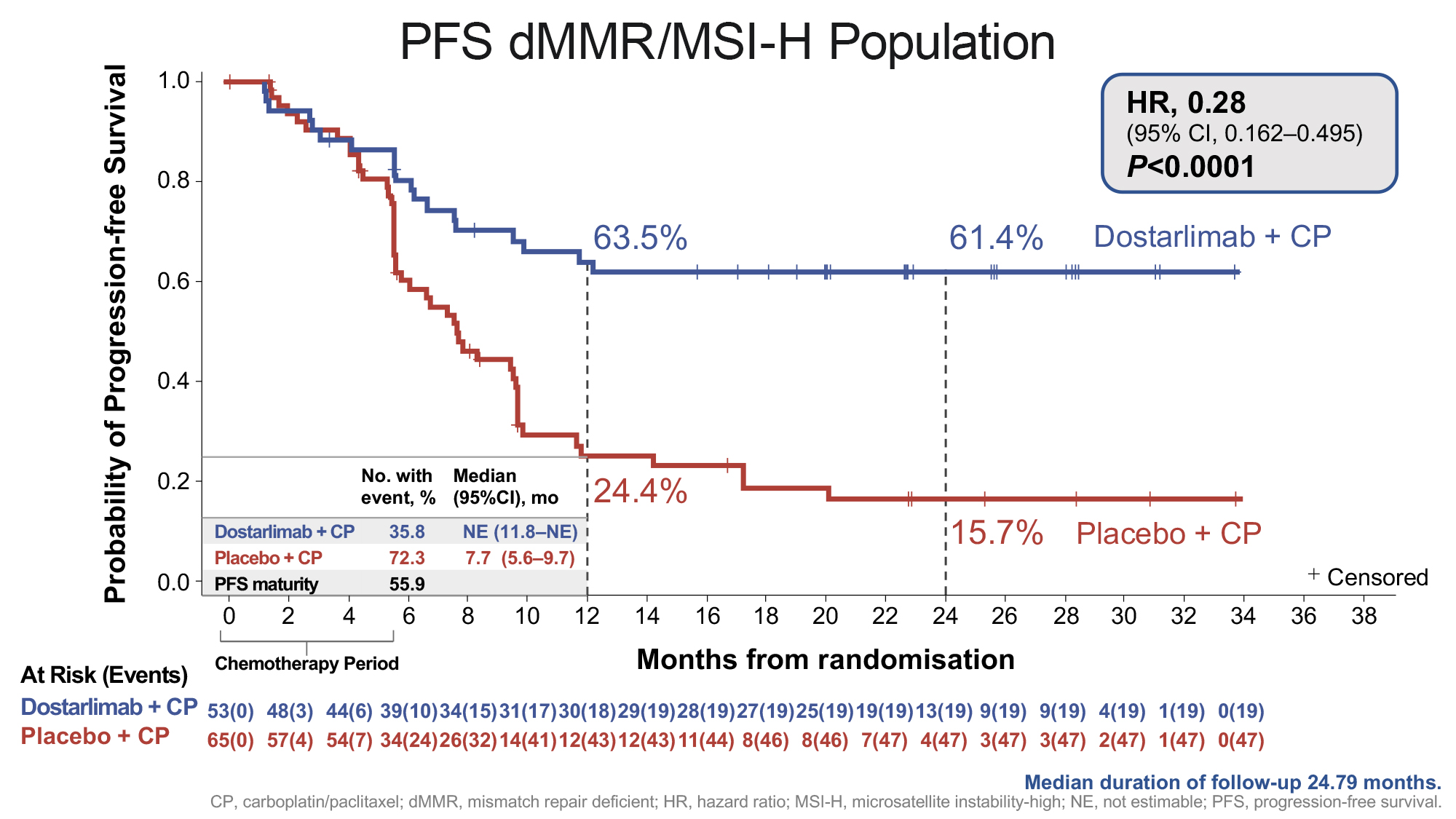
Dr Mansoor Raza Mirza, Chief Oncologist, Copenhagen University Hospital, Denmark and RUBY principal investigator, said: “Clinical practice has been waiting decades for a meaningful advancement in the standard of care for primary advanced or recurrent endometrial cancer. The results from the RUBY clinical trial, especially given the difficult-to-treat histologies included in the trial, demonstrate support for a new treatment standard with the addition of dostarlimab to current standard-of-care chemotherapy.”
Additionally, at this first interim analysis, there was a clinically meaningful overall survival (OS) trend in the overall population among patients receiving dostarlimab plus chemotherapy followed by dostarlimab. The analysis was done at 33% maturity and statistical significance was not reached. OS follow-up continues and further analysis is planned. PFS and OS summaries are listed below.
| dostarlimab + chemotherapy | placebo + chemotherapy | |
|---|---|---|
| PFS dMMR/MSI-H population | ||
| Number of patients evaluated | 53 | 65 |
| HR (95% CI) |
0.28 |
|
| P-value | P<0.0001 | |
| At 24 months (95% CI) |
61.4% |
15.7% |
| PFS overall patient population | ||
| Number of patients evaluated | 245 | 249 |
| HR (95% CI) | 0.64 (0.507–0.800) |
|
| P-value | P<0.0001 | |
| At 24 months (95% CI) |
36.1% |
18.1% |
| PFS mismatch repair proficient (MMRp)/microsatellite stable (MSS) populationa | ||
| Number of patients evaluated | 192 | 184 |
| HR (95% CI) | 0.76 (0.592–0.981) |
|
| P-value | N/A | |
| At 24 months (95% CI) |
28.4% |
18.8% |
| OS overall patient populationb | ||
| HR (95% CI) | 0.64 (0.464–0.870) |
|
| P-value |
P=0.0021c |
|
| At 24 months (95% CI) |
71.3% |
56.0% (48.9–62.5) |
| OS dMMR/MSI-H populationa,d | ||
| HR (95% CI) |
0.30 |
|
| P-value | N/A | |
| At 24 months (95% CI) |
83.3% |
58.7% |
| OS MMRp/MSS populationa,e | ||
| HR (95% CI) | 0.73 (0.515–1.024) |
|
| P-value | N/A | |
| At 24 months (95% CI) |
67.7% |
55.1% |
aExploratory analyses of PFS in MMRp/MSS, OS in dMMR/MSI-H, and OS in MMRp/MSS populations were pre-specified with no planned hypothesis testing. bMaturity ≈33%. cP-value of ≤0.00177 was required for statistical significance at this OS interim analysis. dMaturity ≈26%. eMaturity ≈36%.
The safety and tolerability profile of dostarlimab in combination with carboplatin-paclitaxel in the RUBY phase III trial was generally consistent with the known safety profiles of the individual agents. The most common (>45%) treatment-emergent adverse events (TEAEs) in both treatment arms in the dMMR/MSI-H and overall populations were nausea, alopecia and fatigue, as well as anaemia in the placebo plus chemotherapy arm in the dMMR/MSI-H population. Severe and serious TEAEs were approximately 10% higher in the dostarlimab plus carboplatin-paclitaxel arm compared with the placebo plus carboplatin-paclitaxel arm in the overall population. The nature and types of immune-related adverse events (irAEs) in the dostarlimab plus chemotherapy safety profile were consistent with the mechanism of action of dostarlimab and similar to those reported for other PD-(L)1 inhibitors. In the overall population, 38.2% of participants in the dostarlimab plus carboplatin-paclitaxel arm and 15.4% of participants in the placebo plus carboplatin-paclitaxel arm had irAEs assessed by the investigator as related to dostarlimab or placebo, respectively. The most frequently reported dostarlimab-related irAE categories were endocrinopathies (15.8% dostarlimab-related versus 3.3% placebo-related) and skin adverse reactions (14.1% dostarlimab-related versus 3.7% placebo-related). Discontinuation of dostarlimab or placebo due to a TEAE occurred in 17.4% of patients in the dostarlimab plus chemotherapy treatment arm and 9.3% of patients in the placebo plus chemotherapy treatment arm in the overall population.
These data were shared in a European Society for Medical Oncology (ESMO) Virtual Plenary, presented in a late breaking session at the Society of Gynecologic Oncology (SGO) Annual Meeting on Women’s Cancer (25-28 March) and published simultaneously in The New England Journal of Medicine.
RUBY is part of an international collaboration between the European Network of Gynaecological Oncological Trial groups (ENGOT), a research network of the European Society of Gynaecological Oncology (ESGO) that consists of 22 trial groups from 31 European countries that perform cooperative clinical trials; the GOG Foundation, a non-profit organisation dedicated to transforming the standard of care in gynaecologic oncology; and the Nordic Society of Gynaecological Oncology – Clinical Trial Unit (NSGO-CTU), a non-profit organisation aiming to improve the practice of prevention, diagnosis and treatment for gynaecological cancers by supporting research and conducting clinical trials across countries.
GSK’s ambition is for dostarlimab to become the backbone of the Company’s ongoing immuno-oncology-based research and development programme when used alone and in combination with standard of care and future novel cancer therapies, particularly for patients who currently have limited treatment options. Dostarlimab is being investigated in registrational enabling studies as monotherapy and as part of combination regimens, including in patients with primary advanced or recurrent endometrial cancer, patients with Stage III or IV non-mucinous epithelial ovarian cancer, and patients with other advanced solid tumours or metastatic cancers.
About endometrial cancer
Endometrial cancer is found in the inner lining of the uterus, known as the endometrium. Endometrial cancer is the most common gynaecologic cancer in developed countries, with approximately 417,000 new cases reported each year worldwide[i], and incidence rates are expected to rise by almost 40% by 2040.[ii][iii] Approximately 15-20% of patients with endometrial cancer will be diagnosed with advanced disease at the time of diagnosis.[iv]
About RUBY
RUBY is a two-part global, randomised, double-blind, multicentre phase III trial of patients with primary advanced or recurrent endometrial cancer. Part 1 is evaluating dostarlimab plus carboplatin-paclitaxel followed by dostarlimab versus carboplatin-paclitaxel plus placebo followed by placebo. Part 2 is evaluating dostarlimab plus carboplatin-paclitaxel followed by dostarlimab plus niraparib versus placebo plus carboplatin-paclitaxel followed by placebo. The primary endpoints in Part 1 are investigator-assessed PFS based on the Response Evaluation Criteria in Solid Tumours v1.1 and OS. The statistical analysis plan included pre-specified analyses of PFS in the dMMR/MSI-H and ITT populations and OS in the overall population. Pre-specified exploratory analyses of PFS in the MMRp/MSS population and OS in the dMMR/MSI-H populations were also performed. RUBY Part 1 included a broad population, including histologies often excluded from clinical trials and had approximately 10% of patients with carcinosarcoma and 20% with serous carcinoma. In Part 2, the primary endpoint is investigator-assessed PFS. Secondary endpoints in Part 1 and Part 2 include PFS per blinded independent central review, overall response rate, duration of response, disease control rate, patient-reported outcomes, and safety and tolerability.
About Jemperli (dostarlimab)
Jemperli is a programmed death receptor-1 (PD-1)-blocking antibody that binds to the PD-1 receptor and blocks its interaction with the PD-1 ligands PD-L1 and PD-L2.[v]
Jemperli is not approved anywhere in the world for use in combination with standard-of-care chemotherapy (carboplatin-paclitaxel) followed by dostarlimab for primary advanced or recurrent endometrial cancer. In the US, Jemperli is indicated for adult patients with mismatch repair-deficient (dMMR) recurrent or advanced endometrial cancer, as determined by a US FDA-approved test, that has progressed on or following a prior platinum-containing regimen in any setting and are not candidates for curative surgery or radiation. Jemperli is also indicated in the US for patients with dMMR recurrent or advanced solid tumours, as determined by a US FDA-approved test, that have progressed on or following prior treatment and who have no satisfactory alternative treatment options. The latter indication is approved in the US under accelerated approval based on tumour response rate and durability of response. Continued approval for this indication in solid tumours may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
Jemperli was discovered by AnaptysBio, Inc. and licensed to TESARO, Inc., under a collaboration and exclusive license agreement signed in March 2014. The collaboration has resulted in three monospecific antibody therapies that have progressed into the clinic. These are: Jemperli (GSK4057190), a PD-1 antagonist; cobolimab, (GSK4069889), a TIM-3 antagonist; and GSK4074386, a LAG-3 antagonist. GSK is responsible for the ongoing research, development, commercialisation, and manufacturing of each of these medicines under the agreement.
Important Information for Jemperli in the EU
Indication
Dostarlimab is indicated as monotherapy for the treatment of adult patients with mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H) recurrent or advanced endometrial cancer that has progressed on or following prior treatment with a platinum-containing regimen.
Refer to the Jemperli EMA Reference Information for a full list of adverse events and the complete important safety information in the EU.
GSK in oncology
GSK is committed to maximising patient survival through transformational medicines. GSK’s pipeline is focused on immuno-oncology, tumour cell targeting therapies and synthetic lethality. Our goal is to achieve a sustainable flow of new treatments based on a diversified portfolio of investigational medicines utilising modalities such as small molecules, antibodies, and antibody-drug conjugates, either alone or in combination.
About GSK
GSK is a global biopharma company with a purpose to unite science, technology, and talent to get ahead of disease together. Find out more at gsk.com/company
Cautionary statement regarding forward-looking statements
GSK cautions investors that any forward-looking statements or projections made by GSK, including those made in this announcement, are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Such factors include, but are not limited to, those described under Item 3.D 'Risk factors” in the company's Annual Report on Form 20-F for 2022, GSK’s Q4 Results for 2022 and any impacts of the COVID-19 pandemic.
References
[i] Faizan U, Muppidi V. Uterine Cancer. [Updated 2022 Sep 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available at: https://www.ncbi.nlm.nih.gov/books/NBK562313/.
[ii] Braun MM, et al. Am Fam Physician. 2016;93(6):468-474.
[iii] International Research on Cancer. Global Cancer Observatory. Cancer Tomorrow. https://gco.iarc.fr/tomorrow/en/dataviz/. Accessed 13 July 2022.
[iv] Kantar Health, Cust Study (2018).
[v] Laken H, Kehry M, Mcneeley P, et al. Identification and characterization of TSR-042, a novel anti-human PD-1 therapeutic antibody. European Journal of Cancer. 2016;69,S102. doi:10.1016/s0959-8049(16)32902-1.



